Introduction to Flow Cytometry
- Flow cytometry is a powerful analytical technique widely used in biology, medicine, and research labs to study cells and particles in a fluid suspension.
- It is a laser-based technology that measures physical and chemical characteristics such as:
- Cell size
- Cell shape and granularity (internal complexity)
- Fluorescence intensity (molecular markers)
- Unlike traditional microscopy, flow cytometry can analyze thousands of cells per second, making it fast, accurate, and highly reliable.
- Interestingly, despite its name, a flow cytometer doesn’t only analyze cells – it can also examine chromosomes, microorganisms, or other microscopic particles suspended in fluid.
In simple terms: Flow cytometry is like a cell scanner that passes cells through a laser beam, reads their characteristics, and gives detailed information about each one.
Principle of Flow Cytometry
The principle of flow cytometry is based on the interaction of cells/particles with laser light as they flow in a single-cell suspension. The scattered and emitted light is collected, converted into electronic signals, and analyzed by a computer.
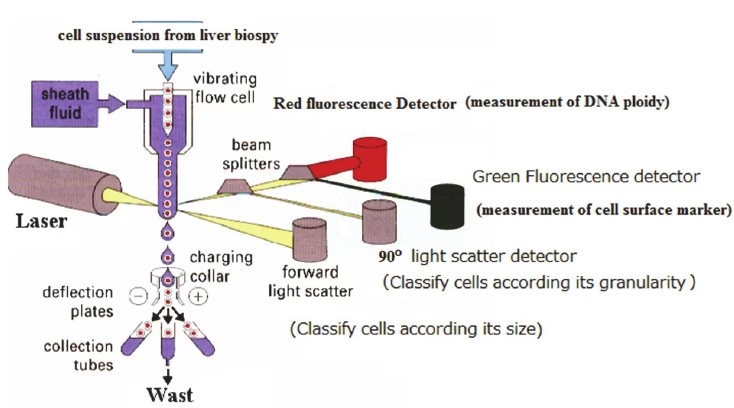
1. Light Scattering
- When a laser hits a cell, it is scattered in different directions:
- Forward Scatter (FSC): Proportional to cell size.
- Side Scatter (SSC): Indicates granularity or internal complexity of the cell.
- Together, FSC and SSC help differentiate cell populations (e.g., lymphocytes vs granulocytes).
2. Fluorescence Detection
- Cells are stained with fluorescent dyes or antibodies conjugated with fluorochromes.
- These fluorochromes absorb laser energy and re-emit it as fluorescence.
- Each fluorochrome emits light at a specific wavelength → detectors identify different molecules simultaneously.
This combination of light scatter + fluorescence allows flow cytometry to study multiple characteristics of thousands of cells in real time.
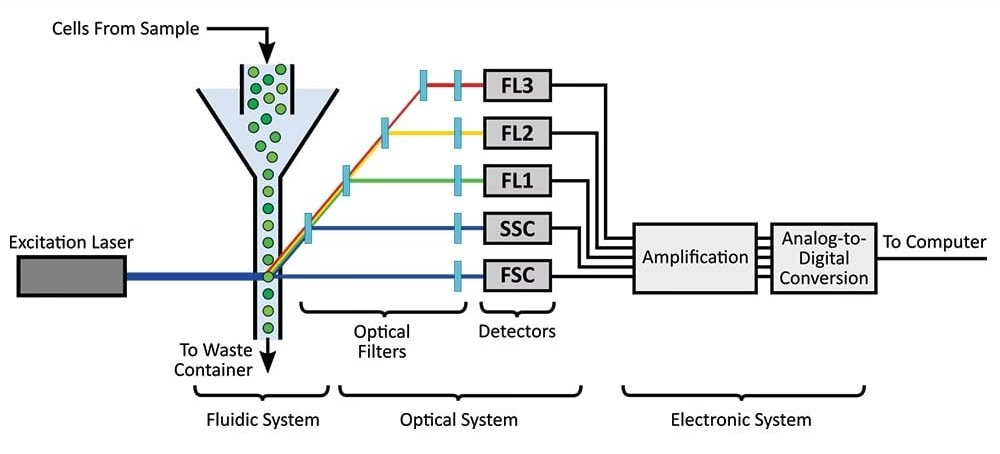
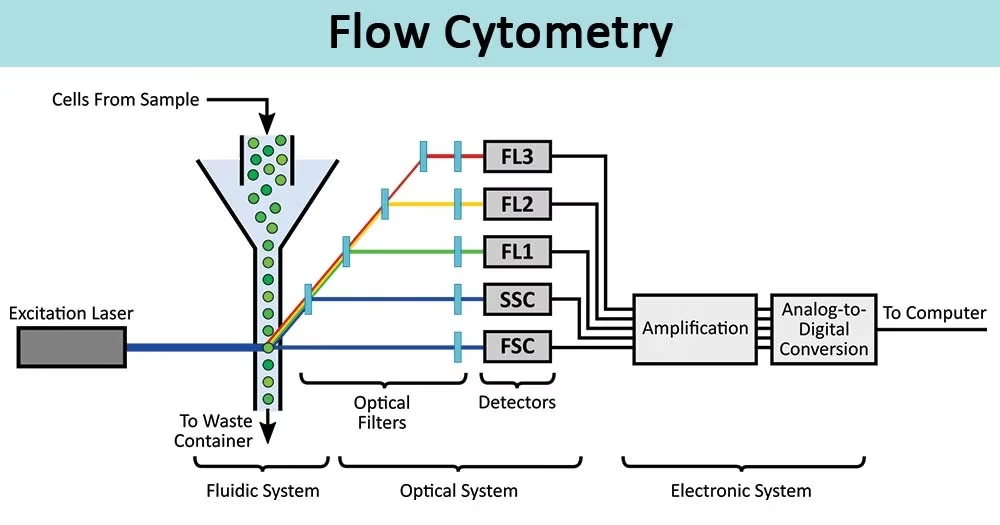
Components / Parts of Flow Cytometer
Flow cytometers have three major systems:
1. Fluidics System
- Transports cells in a fluid stream to the laser.
- Uses sheath fluid (buffered saline) to focus cells into a single file line (hydrodynamic focusing).
- Ensures each cell passes individually through the laser beam.
2. Optics System
- Includes excitation optics (lasers and lenses) and collection optics (mirrors, filters, detectors).
- Lasers excite fluorochromes.
- Detectors capture scattered light and fluorescence.
- Filters ensure only specific wavelengths reach each detector.
3. Electronics System
- Converts optical signals into electronic data.
- Light → electrical pulses → digital signals → displayed as histograms or dot plots.
- Software processes the data to identify and quantify different cell populations.
Steps in Flow Cytometry
1. Sample Preparation
- Cells must be in single-cell suspension.
- Solid tissues are dissociated enzymatically or mechanically.
- Filtration removes clumps and debris.
2. Antibody Staining
- Cells are treated with fluorescently conjugated antibodies specific to cell surface or intracellular markers.
- Types of staining:
- Direct staining – antibody directly linked to fluorochrome.
- Indirect staining – primary antibody detected by fluorochrome-labeled secondary antibody.
- Intracellular staining – requires permeabilization of cells.
3. Running the Sample
- Control samples are run first to set instrument parameters.
- Sample is introduced into the cytometer.
- Cells pass through the laser one by one, and signals are recorded.
4. Data Collection & Analysis
- Software generates plots:
- Histograms (one parameter vs cell count).
- Dot plots/scatter plots (two parameters).
- Researchers interpret cell size, granularity, and marker expression.
Types of Flow Cytometry
- Traditional Flow Cytometers
- Use sheath fluid for hydrodynamic focusing.
- Lasers: blue (488 nm), red (640 nm), violet (405 nm), etc.
- Acoustic Focusing Cytometers
- Use ultrasonic waves instead of sheath fluid for focusing.
- Reduce clogging and allow higher sample throughput.
- Cell Sorters (FACS – Fluorescence-Activated Cell Sorting)
- Special cytometers that can separate and collect specific cells after analysis.
- Widely used in immunology and stem cell research.
- Imaging Flow Cytometers
- Combine flow cytometry with fluorescence microscopy.
- Provide both morphological images and fluorescence data at single-cell resolution.
Applications of Flow Cytometry
Flow cytometry is one of the most versatile tools in biology and medicine:
1. Clinical Applications
- Diagnosis of leukemia and lymphoma.
- Monitoring HIV infection (CD4/CD8 counts).
- Detection of immunodeficiencies.
- Analysis of blood cancers and other hematological disorders.
2. Research Applications
- Studying cell cycle phases using DNA-binding dyes.
- Detecting apoptosis (cell death) and necrosis.
- Characterizing immune cell subtypes.
- Tracking gene expression with fluorescent reporters.
3. Biotechnology & Pharma
- Quality control in vaccine production.
- Screening new drug effects on cells.
- Sorting specific cells for stem cell therapies.
4. Environmental & Agricultural Uses
- Detecting microorganisms in water.
- Analyzing plant cell populations.
- Studying marine biology samples.
Advantages of Flow Cytometry
- Analyzes thousands of cells within seconds.
- Provides multi-parameter analysis at single-cell level.
- High sensitivity and accuracy.
- Can sort and isolate specific cells (FACS).
- Applicable to wide fields: medicine, research, environment.
Limitations of Flow Cytometry
- Expensive instruments and maintenance.
- Requires skilled operators.
- Cannot provide intracellular location of proteins.
- Sample preparation is time-consuming.
- Debris or aggregates may cause false results.
Conclusion
- Flow cytometry is a cutting-edge cell analysis tool that has transformed clinical diagnostics, biomedical research, and biotechnology.
- Its ability to analyze thousands of cells in seconds with high accuracy makes it a gold standard in cell biology.
- Although expensive and technically demanding, its applications in cancer research, immunology, virology, and stem cell biology ensure its continued importance in science and medicine.
In short: Flow cytometry is the microscope of the future, offering speed, precision, and deep insights into cellular life.
Frequently Asked Questions (FAQs) on Flow Cytometry
Q1. What is flow cytometry?
Ans: Flow cytometry is a laser-based technology that analyzes the physical and chemical properties of cells or particles as they flow in a fluid stream through a laser beam.
Q2. Who invented flow cytometry?
Ans: The concept was developed in the 1960s by Mack Fulwyler, often called the father of flow cytometry.
Q3. What is the principle of flow cytometry?
Ans: Flow cytometry works on the principle that cells scatter light and emit fluorescence when excited by a laser. The scattered and emitted light provides information about cell size, granularity, and molecular markers.
Q4. What does FSC and SSC mean in flow cytometry?
Ans:
- FSC (Forward Scatter): Proportional to cell size.
- SSC (Side Scatter): Indicates cell granularity/internal complexity.
Q5. What is FACS in flow cytometry?
Ans: FACS (Fluorescence-Activated Cell Sorting) is a special type of flow cytometry that not only analyzes cells but also sorts and collects specific populations for further study.
Q6. What are fluorochromes in flow cytometry?
Ans: Fluorochromes are fluorescent dyes or antibody conjugates that bind to target molecules. They absorb laser energy and re-emit light at specific wavelengths, allowing identification of proteins or nucleic acids.
Q7. What are the main components of a flow cytometer?
Ans:
- Fluidics system – transports cells in a single stream.
- Optics system – lasers and detectors capture light signals.
- Electronics system – converts signals into digital data.
Q8. What type of samples can be analyzed by flow cytometry?
Ans: Blood, bone marrow, cultured cells, bacteria, yeast, stem cells, plant cells, and even microorganisms in water can be analyzed.
Q9. Can flow cytometry measure DNA content?
Ans: Yes. By using DNA-binding dyes, flow cytometry measures cell cycle phases, ploidy, and apoptosis.
Q10. What diseases are diagnosed using flow cytometry?
Ans:
- Leukemia and lymphoma
- HIV (CD4/CD8 counts)
- Immunodeficiencies
- Blood cancers and immune disorders
Q11. What is the advantage of flow cytometry over microscopy?
Ans: Unlike microscopy, flow cytometry analyzes thousands of cells per second, provides quantitative data, and allows multi-parameter analysis.
Q12. What is the difference between flow cytometry and hematology analyzers?
Ans: Hematology analyzers give general counts of blood cells, while flow cytometry provides detailed immunophenotyping and molecular profiling.
Q13. Is flow cytometry quantitative or qualitative?
Ans: Flow cytometry provides both qualitative (presence/absence of markers) and quantitative (intensity, percentage of cells) information.
Q14. What is the role of compensation in flow cytometry?
Ans: Compensation corrects spectral overlap when multiple fluorochromes emit at similar wavelengths, ensuring accurate results.
Q15. How fast is flow cytometry?
Ans: A flow cytometer can analyze 10,000–50,000 cells per second.
Q16. What is the importance of controls in flow cytometry?
Controls (unstained, single-stained, isotype) are crucial to set instrument parameters, verify accuracy, and avoid false positives.
Q17. What is gating in flow cytometry?
Ans: Gating is the process of selecting specific populations of cells from a graph (dot plot or histogram) for detailed analysis.
Q18. Can flow cytometry be used in vaccine development?
Ans: Yes. It is widely used in vaccine quality control, immune response monitoring, and antigen-specific cell detection.
Q19. What is imaging flow cytometry?
Ans: A hybrid method that combines microscopy with flow cytometry, giving both morphological images and fluorescence data at the single-cell level.
Q20. What are the advantages of flow cytometry?
Ans:
- High speed and sensitivity
- Multi-parameter single-cell analysis
- Ability to sort specific cells (FACS)
- Wide clinical and research applications
Q21. What are the limitations of flow cytometry?
Ans:
- High cost of instruments and reagents
- Requires skilled personnel
- Complex sample preparation
- Cannot provide intracellular localization of molecules
Q22. Can flow cytometry analyze dead cells?
Ans: Dead cells can produce false signals. Hence, viability dyes (like propidium iodide) are used to exclude dead cells from analysis.
- https://www.bosterbio.com/protocol-and-troubleshooting/flow-cytometry-principle
- http://www.bu.edu/flow-cytometry/files/2010/10/BD-Flow-Cytom-Learning-Guide.pdf
- https://microbenotes.com/flow-cytometry/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5939936/
- https://www.slideshare.net/Raghuveer19/flow-cytometry-73042192
- https://www.slideshare.net/PradeepNarwat/flow-cytometry-152440593
- https://www.amrepflow.org.au/public/documents/SyllabusAMREPFlowCoreFlowCytometry160120.pdf
- https://docs.abcam.com/pdf/protocols/Introduction_to_flow_cytometry_May_10.pdf
- http://www.rsc.org/suppdata/c7/sc/c7sc00260b/c7sc00260b1.pdf
- http://site.iugaza.edu.ps/hesawwaf/files/2016/09/Lab.5-immunofluorescence1.pptx
- https://www.usbio.net/promos/flow-cytometry
Other related topics you might be interested in:
Autoclave – Principle, Parts, Procedure, Types, Uses, Advantages, Limitations
Bioreactor – Principle, Design, Parts, Types, Applications, and Limitations
Bunsen Burner – Principle, Parts, Types, Flames, Applications, Advantages & Precautions
Centrifuge – Principle, Parts, Types, Operation, Applications and Advantages
Colony Counter – Definition, Principle, Types, Parts, Working, Applications, Advantages & Examples
Fluorimetry – Principle, Instrumentation, Factors, Applications, Advantages & Limitations
Homogenizer – Principle, Parts, Types, Working, Procedure, Applications, Advantages & Limitations
Hot Air Oven – Principle, Parts, Types, Working, Applications & Advantages
Instruments Used in Microbiology Laboratory – Principles, Uses, and Applications
Laboratory Incubator – Principle, Types, Components, Working, Applications, Advantages & Limitations
Laminar Flow Hood / Cabinet – Principle, Types, Parts, Procedure, Applications & Precautions
Micropipette – Definition, Types, Parts, Working, Applications, Errors, Calibration & Limitations
pH Meter – Principle, Parts, Working, Procedure, Types, Applications, Advantages & Precautions
Pipettes – Principle, Types, Uses, Parts, Operation, Advantages & Precautions
Ultracentrifuge – Principle, Types, Parts, Working Procedure, Applications, Advantages & Precautions
Vortex Mixer – Definition, Principle, Parts, Types, Working, Applications, Advantages & Precautions