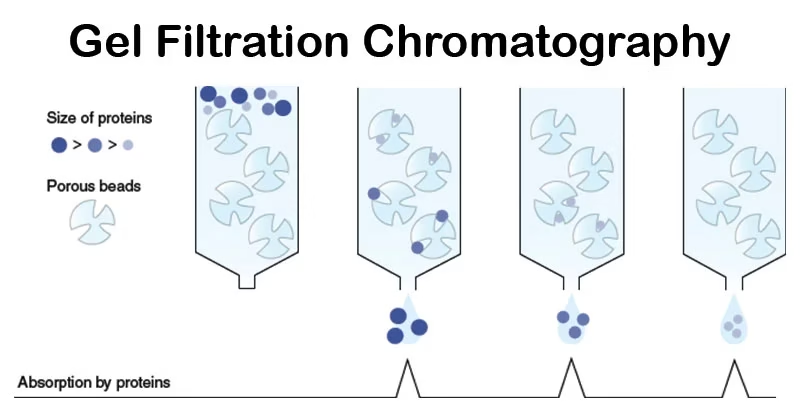
Introduction to Gel Filtration Chromatography
- Gel Filtration Chromatography (GFC), also called Size Exclusion Chromatography (SEC) or Molecular Sieve Chromatography, is one of the simplest and mildest techniques to separate biomolecules.
- Separation is purely based on size or molecular weight, unlike other chromatographic methods that rely on charge, hydrophobicity, or affinity.
- GFC is widely used for the purification of proteins, enzymes, nucleic acids, polysaccharides, and synthetic polymers.
- It is highly valued because it does not denature or chemically alter the molecules being separated.
In simple terms: Gel Filtration Chromatography separates molecules like a sieve – larger molecules pass through quickly, while smaller ones take longer paths through the pores of the gel beads.
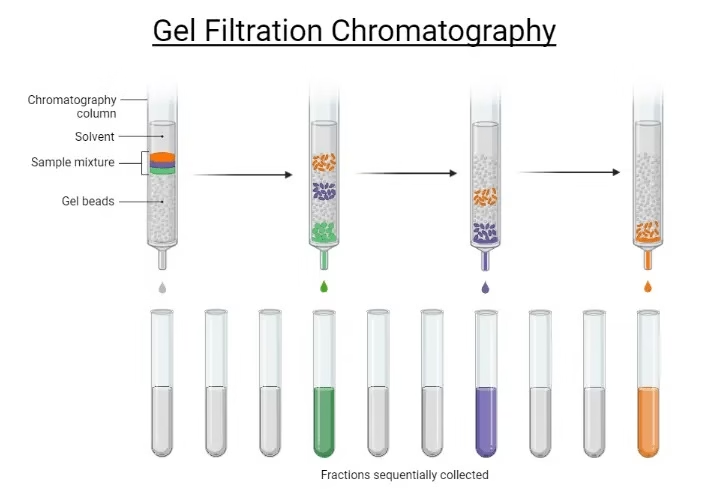
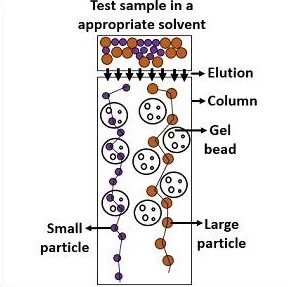
Principle of Gel Filtration Chromatography
- The stationary phase is made of porous polymer beads (gel matrix), which are chemically inert and stable.
- The mobile phase is a buffer solution that flows continuously through the column.
- When the sample mixture is applied:
- Large molecules → cannot enter the pores → move around the beads → elute first.
- Small molecules → diffuse into the pores → travel a longer path → elute later.
Thus, separation occurs solely due to size differences, without any binding between the sample and the matrix.
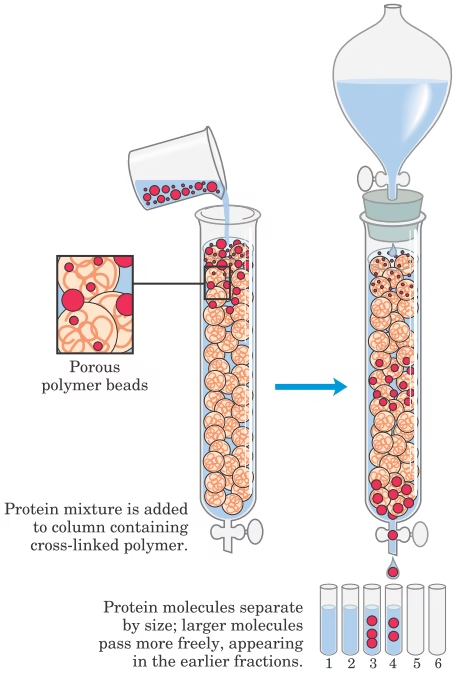
Types of Gel Filtration Chromatography
Gel filtration can be broadly categorized into two types depending on the goal of separation:
1. Group Separation
- Molecules are separated into two broad groups (large vs small).
- Used for:
- Buffer exchange
- Desalting proteins
- Removing contaminants like phenol red from culture fluids
2. High-Resolution Fractionation
- Provides detailed separation of biomolecules by size differences.
- Used for:
- Isolation of monomers from aggregates
- Molecular weight determination
- Molecular weight distribution analysis
- Fractionation of proteins, polysaccharides, and nucleic acids
Components of Gel Filtration Chromatography
- Stationary Phase (Gel Beads)
- Made of porous materials such as Sephadex (dextran), Sepharose (agarose), Bio-Gel (polyacrylamide), or cross-linked polystyrene.
- Chemically inert, hydrophilic, and stable.
- Column
- Cylindrical tube filled with gel beads.
- Available in different lengths and diameters depending on analytical or preparative purposes.
- Mobile Phase (Buffer)
- Provides constant flow through the column.
- Maintains biomolecule stability.
- Composition depends on the sample (aqueous buffers for proteins, organic solvents for polymers).
- Pump System
- Maintains steady flow rate.
- Essential for reproducible results.
- Detectors
- Measure molecules as they elute from the column.
- Types:
- UV detector – for proteins and nucleic acids
- Refractive index detector – universal detection
- Fluorescence detector – for labeled molecules
- Recorder/Software
- Converts signals into a chromatogram.
- Provides data on retention time, molecular weight, and concentration.
Steps in Gel Filtration Chromatography
Step 1. Column Packing
- Gel beads are swollen in buffer and packed into the column.
Step 2. Equilibration
- The column is equilibrated with buffer to ensure uniform conditions.
Step 3. Sample Application
- The sample mixture (proteins, enzymes, or polymers) is applied carefully to avoid disturbance.
Step 4. Elution
- The mobile phase carries molecules through the column.
- Larger molecules elute faster, smaller ones are delayed.
Step 5. Detection
- Eluted molecules are detected, and a chromatogram is recorded.
Step 6. Data Analysis
- Peak positions → molecular size.
- Peak area → concentration.
Applications of Gel Filtration Chromatography
- Protein Purification
- Separation of enzymes, antibodies, and structural proteins.
- Nucleic Acid Studies
- Separation of RNA, DNA fragments, and nucleotides.
- Polysaccharide Analysis
- Fractionation of starch, cellulose derivatives, and glycans.
- Molecular Weight Determination
- Estimation of native molecular weight of biomolecules.
- Buffer Exchange and Desalting
- Removal of salts, detergents, and small molecules from protein samples.
- Refolding of Denatured Proteins
- Controlled buffer changes allow proteins to regain their natural structure.
- Quaternary Structure Analysis
- Determining oligomeric states of proteins (monomer, dimer, trimer, etc.).
- Polymer Science
- Used to study synthetic polymers and determine polydispersity.
Advantages of Gel Filtration Chromatography
- Gentle method – no harsh chemicals required.
- Molecules do not bind to the stationary phase.
- Can handle delicate biomolecules.
- Fast separation with reproducible results.
- Works in a wide range of pH and ionic strengths.
- No sample loss during process.
- Requires small amounts of solvent.
Limitations of Gel Filtration Chromatography
- Limited resolution if molecules are too similar in size.
- Requires pre-filtration to avoid column clogging.
- Expensive gels and columns.
- Cannot separate molecules of same size but different shapes.
- Broad peaks when molecules have very close molecular masses.
Conclusion
- Gel Filtration Chromatography (GFC) is one of the most reliable and widely used techniques for separating biomolecules by size.
- Its non-destructive nature, reproducibility, and versatility make it invaluable in biochemistry, biotechnology, and medicine.
- Though limited in resolution for closely sized molecules, its simplicity and broad applications ensure its continued importance in research and industry.
In short: Gel Filtration Chromatography is the molecular sieve of biochemistry, separating molecules efficiently and gently for further study.
Frequently Asked Questions (FAQs) on Gel Filtration Chromatography
Q1. What is Gel Filtration Chromatography (GFC)?
Ans: GFC is a size-based separation technique that uses a porous gel matrix to separate molecules according to their molecular size and weight.
Q2. Is Gel Filtration the same as Size Exclusion Chromatography (SEC)?
Ans: Yes. GFC is a type of Size Exclusion Chromatography. The terms are often used interchangeably in biological research.
Q3. What is the difference between Gel Filtration and Gel Permeation Chromatography (GPC)?
Ans: Both are based on size exclusion:
- GFC is mainly used for biological molecules in aqueous solutions.
- GPC is typically used for synthetic polymers in organic solvents.
Q4. What is the principle of Gel Filtration Chromatography?
Ans: Molecules are separated according to their ability to enter the pores of the gel beads:
- Large molecules → cannot enter pores → elute first.
- Small molecules → enter pores → travel longer → elute later.
Q5. What are the stationary phases used in GFC?
Ans: Common gel matrices include:
- Sephadex (dextran-based)
- Sepharose (agarose-based)
- Bio-Gel (polyacrylamide-based)
- Cross-linked polystyrene
Q6. What is the mobile phase in Gel Filtration Chromatography?
Ans: The mobile phase is usually a buffer solution (for proteins and nucleic acids) or organic solvents (for synthetic polymers).
Q7. What detectors are used in GFC?
Ans:
- UV detector – for proteins and nucleic acids.
- Refractive index detector (RI) – universal.
- Fluorescence detector – for labeled molecules.
Q8. Why do large molecules elute first in GFC?
Ans: Large molecules cannot penetrate the pores of the gel beads, so they travel around them and come out of the column faster.
Q9. What is the void volume in GFC?
Ans: Void volume (Vo) is the volume of solvent outside the gel beads, where only large molecules can travel.
Q10. What are the applications of Gel Filtration Chromatography?
Ans:
- Protein purification
- Enzyme and antibody isolation
- Molecular weight determination
- Buffer exchange and desalting
- Quaternary structure analysis of proteins
- Separation of nucleic acids and polysaccharides
- Polymer analysis in material science
Q11. What are the advantages of Gel Filtration Chromatography?
Ans:
- Gentle and non-destructive
- Reproducible and simple
- Works in a wide range of pH and ionic strengths
- No sample binding to the matrix
- Compatible with delicate biomolecules
Q12. What are the limitations of GFC?
Ans:
- Expensive gels and columns
- Limited resolution if molecules are close in size
- Requires pre-filtration to prevent clogging
- Cannot separate molecules of the same size but different shapes
Q13. Is Gel Filtration Chromatography quantitative or qualitative?
Ans: It can be both:
- Qualitative: shows molecular size differences.
- Quantitative: determines molecular weight and concentration using standards.
Q14. Can GFC be used for desalting proteins?
Ans: Yes. GFC is widely used to remove salts, detergents, and small contaminants from protein samples.
Q15. Can GFC be used for nucleic acid separation?
Ans: Yes. It can separate RNA, DNA fragments, and nucleotides by size.
Q16. What is the difference between GFC and Ion Exchange Chromatography?
Ans:
- GFC: separates by size.
- Ion Exchange Chromatography: separates by charge.
Q17. What is the difference between GFC and Affinity Chromatography?
Ans:
- GFC: based on molecular size.
- Affinity Chromatography: based on specific interactions (e.g., antigen–antibody).
Q18. What is the resolution of GFC?
Ans: Resolution depends on pore size distribution, column length, flow rate, and sample loading. High-resolution gels give sharper separations.
Q19. Can GFC determine protein quaternary structure?
Ans: Yes. GFC helps estimate whether a protein exists as a monomer, dimer, trimer, or higher oligomer.
Q20. What industries use Gel Filtration Chromatography?
Ans:
- Biotechnology – protein purification
- Pharmaceuticals – drug formulation analysis
- Food industry – analysis of polysaccharides
- Polymer industry – quality control and molecular weight distribution
Q21. How does flow rate affect Gel Filtration Chromatography?
Ans:
- Too high flow rate: poor separation and band broadening.
- Too low flow rate: long analysis time.
*Optimal flow rate ensures high resolution and accuracy.
Q22. Is Gel Filtration Chromatography destructive?
Ans: No. It is a non-destructive method, meaning the molecules retain their native structure and biological activity after separation.
References
- http://kirschner.med.harvard.edu/files/protocols/GE_gelfiltration.pdf
- Wilson, K., Walker, J. (2018). Principles and Techniques of Biochemistry and Molecular Biology (8 eds.). Cambridge University Press: New York.
- https://www.slideshare.net/asabuwangwa/gel-permeation-chromatography-gpc
- https://microbenotes.com/gel-filtration-chromatography/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5206469/
Other related topics you might be interested in:
Chromatography – Principle, Types, Steps, Uses, and Advantages
Adsorption Chromatography – Principle, Types, Procedure, Applications & Advantages
Affinity Chromatography – Principle, Components, Procedure, Applications & Advantages
Gas Chromatography (GC) – Principle, Parts, Procedure, Steps, Applications, Advantages & Limitations
High-Performance Liquid Chromatography (HPLC) – Principle, Instrumentation, Types & Applications
Ion Exchange Chromatography – Principle, Instrumentation, Procedure, Applications
Paper Chromatography – Definition, Principle, Types, Steps, Applications, Advantages & Limitations
Thin Layer Chromatography (TLC) – Principle, Steps, Applications, Advantages & Limitations