What is Column Chromatography?
- Column Chromatography is one of the most important methods used in biochemistry, analytical chemistry, and biotechnology for separating and purifying components of a mixture.
- It works on the basic principle of adsorption or partition between two phases – a stationary phase (solid) and a mobile phase (liquid or gas).
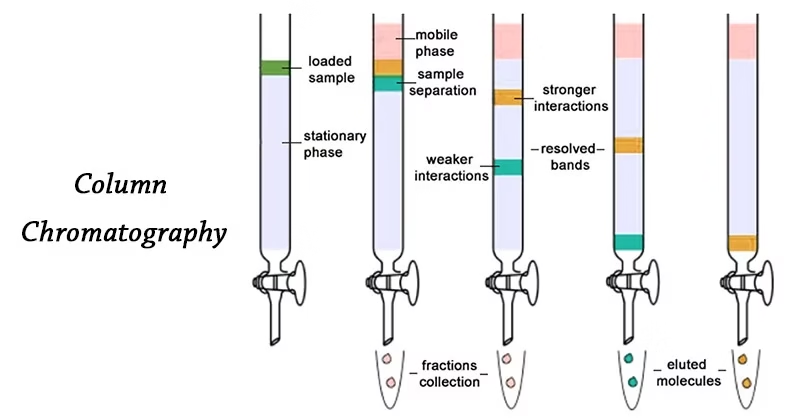
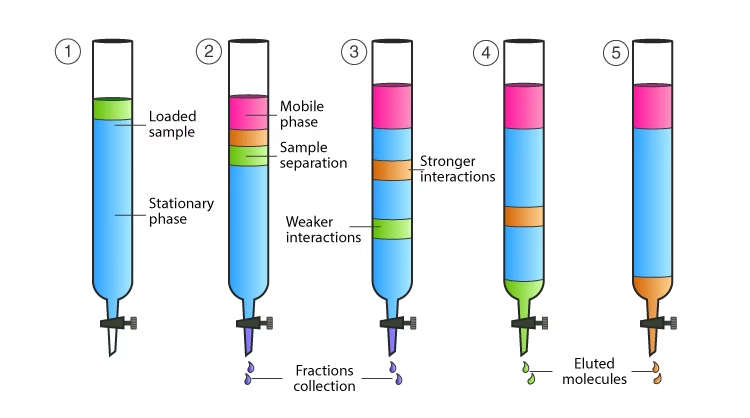
- The mixture to be separated is placed at the top of a column packed with an adsorbent. As the mobile phase moves through the column, the compounds in the mixture travel at different speeds based on their affinity toward the stationary and mobile phases.
- Substances that are strongly adsorbed move slowly, while those with weaker interactions move faster, resulting in separation.
In simple terms, column chromatography works like a filter that separates compounds based on how strongly they stick to the column material.
Historical Background
- Column chromatography was first developed by D.T. Day (1900).
- Later, Mikhail Tswett (1906), a Polish botanist, used the method to separate plant pigments (chlorophyll, xanthophyll, carotenoids), which gave birth to the modern form of chromatography.
- Today, it is one of the most widely used laboratory separation techniques for both analytical and preparative purposes.
Principle of Column Chromatography
- Column chromatography is based on the differential adsorption principle.
- The stationary phase (solid) adsorbs different compounds of a mixture to different degrees.
- The mobile phase (liquid or gas) helps move these compounds through the stationary phase.
- Each compound travels through the column at a speed depending on its affinity for the stationary vs. mobile phase.
- Components with low affinity elute first, while those with high affinity take longer.
Simplified Process:
- Sample mixture is applied on top of the column.
- The mobile phase passes through the stationary phase.
- Components move at different rates.
- The separated compounds are collected at the column’s base as fractions.
Parts and Components of a Column Chromatography Setup
A typical column chromatography system includes:
1. Stationary Phase
- Usually a solid adsorbent material like silica gel, alumina, cellulose, or ion-exchange resins.
- Choice of adsorbent depends on the type of compounds to be separated.
- The surface of the adsorbent interacts with sample molecules, enabling separation.
2. Column
- Made of glass or stainless steel, typically 25–50 cm long and 4–10 mm internal diameter.
- It holds the stationary phase and allows the mobile phase to pass through.
- Types:
- Conventional columns: filled with stationary phase.
- Microbore columns: thin film coating inside the column wall (common in gas chromatography).
3. Mobile Phase and Delivery System
- The mobile phase (eluent) can be a single solvent or a mixture.
- It carries the sample through the stationary phase.
- Flow rate is maintained constant using gravity, gas pressure, or a pumping system.
4. Injector System
- Introduces the sample mixture into the top of the column accurately.
- Ensures reproducibility and prevents sample overloading.
5. Detector and Recorder
- Detects the eluted compounds as they come out of the column.
- Common detection methods: UV-visible absorption, fluorescence, or refractive index.
- Data is displayed as chromatographic peaks on a recorder or computer system.
6. Fraction Collector
- Collects separated compounds into individual tubes for further analysis or use.
Steps in Column Chromatography
Column chromatography involves several sequential steps:
A. Preparation of the Column
- Select a clean, dry glass column.
- Place a small piece of cotton wool or glass wool at the bottom to prevent adsorbent loss.
- Fill the column with stationary phase by one of two methods:
i. Dry Packing Method:
- Dry adsorbent powder is poured into the column.
- Solvent is allowed to pass until uniform packing is achieved.
ii. Wet Packing Method:
- A slurry of adsorbent with the mobile phase is prepared and poured into the column.
- Ensures uniform, bubble-free packing (preferred method).
Before use, the column should be washed, dried, and equilibrated with the mobile phase.
B. Sample Application
- The sample mixture (dissolved in minimum solvent) is gently added to the top of the column.
- It gets adsorbed onto the upper surface of the stationary phase.
C. Elution (Separation Process)
This is the main step where compounds move through the column and get separated.
There are two main elution techniques:
i. Isocratic Elution:
- Uses a single solvent or a solvent mixture of constant composition.
- Example: Using chloroform alone throughout.
ii. Gradient Elution:
- Uses a series of solvents of increasing polarity to improve separation.
- Example: Starting with benzene, then chloroform, followed by ethyl acetate.
As elution proceeds, compounds come out of the column at different times and can be collected in test tubes or vials.
D. Detection of Components
- If compounds are colored, they can be seen visually as bands.
- For colorless compounds, fractions are collected and analyzed using Thin Layer Chromatography (TLC) or UV spectroscopy.
Factors Affecting Column Efficiency
Several factors can influence the efficiency and resolution of separation:
- Column Dimensions: Longer columns improve separation but increase time.
- Particle Size: Smaller adsorbent particles increase surface area but slow flow rate.
- Solvent Type: Polarity affects the elution rate and selectivity.
- Flow Rate: Too high a flow rate causes poor separation; too low makes the process slow.
- Temperature: Affects viscosity and diffusion of solvent.
- Pressure: Maintains consistent flow and prevents channeling.
Forms and Types of Column Chromatography
Column chromatography can be classified based on separation mechanism or phase type:
1. Adsorption Chromatography
- Based on differential adsorption of compounds onto the solid stationary phase.
- Common adsorbents: Silica gel, alumina.
2. Partition Chromatography
- Based on partitioning of solutes between stationary liquid and mobile liquid phase.
- Example: Separation of amino acids or small biomolecules.
3. Ion Exchange Chromatography
- Separation based on ionic charge of molecules.
- Stationary phase has charged functional groups.
- Commonly used for proteins and nucleic acids.
4. Gel Filtration (Size Exclusion) Chromatography
- Based on molecular size and shape.
- Large molecules elute first, smaller molecules enter pores and come out later.
5. Affinity Chromatography
- Based on specific biological interactions like enzyme–substrate or antigen–antibody binding.
- Extremely specific and used for purifying proteins and enzymes.
Applications of Column Chromatography
Column chromatography has a wide range of applications in science and industry:
- Separation and purification of organic compounds.
- Isolation of active constituents from plants or biological samples.
- Removal of impurities from crude extracts.
- Purification of enzymes, proteins, and nucleic acids.
- Drug formulation and quality testing in pharmaceuticals.
- Quantitative estimation of compounds in mixtures.
- Isolation of metabolites from biological fluids.
- Environmental testing for pollutants and contaminants.
Advantages of Column Chromatography
- Can separate both solids and liquids.
- Works for any sample size – small analytical to large preparative scales.
- Wide range of solvents can be used.
- Reusable adsorbents in preparative type.
- Can be automated for industrial and high-throughput analysis.
- Non-destructive to many biological samples.
Limitations of Column Chromatography
- Time-consuming method compared to modern techniques.
- Requires large quantities of solvents, which may be expensive.
- Manual operation can be less reproducible.
- Automation increases cost and complexity.
- Skill-dependent for efficient packing and operation.
Conclusion
- Column Chromatography remains a fundamental and versatile separation method in laboratories worldwide.
- It provides high-quality separation and purification of compounds based on their physicochemical properties.
- Despite newer techniques like HPLC, column chromatography is still widely used due to its simplicity, affordability, and effectiveness.
In short: Column chromatography is the backbone of biochemical and analytical separations—simple, efficient, and powerful.
Frequently Asked Questions (FAQs) on Column Chromatography
Q1. What is Column Chromatography?
Ans: Column Chromatography is a laboratory technique used to separate and purify the components of a mixture based on their differential affinities toward a stationary phase (solid) and a mobile phase (liquid).
Q2. Who discovered Column Chromatography?
Ans: The technique was first developed by D.T. Day in 1900 and later improved by Mikhail Tswett in 1906, who used it to separate plant pigments like chlorophyll and carotenoids.
Q3. What is the basic principle of Column Chromatography?
Ans: It works on the adsorption principle—different compounds in a mixture interact differently with the stationary phase and move at different speeds through the column, resulting in separation
Q4. What are the two main phases in Column Chromatography?
Ans:
- Stationary Phase: The solid adsorbent material (e.g., silica gel, alumina).
- Mobile Phase: The liquid solvent or solvent mixture that moves through the column.
Q5. What are common stationary phases used in Column Chromatography?
Ans: Silica gel, alumina, cellulose, ion-exchange resins, and Sephadex (for gel filtration).
Q6. What are the common mobile phases used?
Ans: Solvents like hexane, benzene, chloroform, ethyl acetate, ethanol, methanol, and water, either alone or in combination.
Q7. What is Elution in Column Chromatography?
Ans: Elution is the process of washing down the components through the column using a suitable solvent (mobile phase) to separate and collect them as fractions.
Q8. What are the types of Elution?
Ans:
- Isocratic Elution: Uses a single solvent throughout the process.
- Gradient Elution: Uses a series of solvents with increasing polarity for better separation.
Q9. What is the role of the stationary phase?
Ans: The stationary phase retains or adsorbs certain components more strongly than others, controlling how fast each compound moves through the column.
Q10. What determines the rate of movement of compounds through the column?
Ans: The affinity or interaction strength of each compound with the stationary and mobile phases. Compounds more strongly adsorbed to the stationary phase move slower.
Q11. What are the common adsorbents used for different separations?
Ans:
- Silica gel: For polar compounds.
- Alumina: For weakly polar compounds.
- Cellulose: For biochemical separations.
- Ion exchange resins: For ionic compounds.
Q12. What are the main steps in Column Chromatography?
Ans:
- Preparation of the column.
- Loading the sample.
- Elution of components.
- Detection and collection of fractions.
Q13. What are the different types of Column Chromatography?
Ans:
- Adsorption Chromatography – based on differential adsorption.
- Partition Chromatography – based on solute partition between two liquids.
- Ion Exchange Chromatography – based on ionic charge.
- Gel Filtration Chromatography – based on molecular size.
- Affinity Chromatography – based on specific biological interactions.
Q14. What is the difference between Adsorption and Partition Chromatography?
Ans:
- Adsorption Chromatography: Separation occurs on a solid stationary phase through adsorption.
- Partition Chromatography: Separation is based on the distribution of solutes between two liquid phases.
Q15. How are colorless compounds detected in Column Chromatography?
Ans: By using Thin Layer Chromatography (TLC) or by UV-Visible spectrophotometry after collecting fractions.
Q16. What are the factors that affect column performance?
Ans:
- Column length and diameter.
- Particle size of stationary phase.
- Type and polarity of solvent.
- Flow rate of the mobile phase.
- Temperature and pressure.
Q17. What are the applications of Column Chromatography?
Ans:
- Separation and purification of organic compounds.
- Isolation of plant pigments and natural products.
- Purification of proteins, enzymes, and nucleic acids.
- Detection of drug impurities.
- Environmental and food quality analysis.
Q18. What are the advantages of Column Chromatography?
Ans:
- Simple and versatile technique.
- Can handle large sample volumes.
- Non-destructive and reusable.
- Suitable for analytical and preparative purposes.
- Works for both organic and inorganic compounds.
Q19. What are the limitations of Column Chromatography?
Ans:
- Time-consuming and solvent-intensive.
- Requires skilled handling for consistent results.
- Lower resolution compared to HPLC.
- Difficult for very complex mixtures.
Q20. What is the difference between Column Chromatography and Thin Layer Chromatography (TLC)?
Ans:
| Feature | Column Chromatography | Thin Layer Chromatography |
| Type | Preparative | Analytical |
| Stationary Phase | Packed in column | Thin layer on glass plate |
| Sample Quantity | Large | Very small |
| Visualization | Collected fractions | Direct on plate |
| Time | Slower | Faster |
Q21. Can Column Chromatography be automated?
Ans: Yes. In industrial and research settings, automated column chromatography systems are used for high-throughput purification and reproducibility.
Q22. How does Column Chromatography differ from HPLC?
Ans:
- Column Chromatography: Uses gravity or low pressure; low cost but slower.
- HPLC (High-Performance Liquid Chromatography): Uses high pressure and advanced detectors for faster, high-resolution separations.
Q23. What are the key parameters for optimization in Column Chromatography?
Ans: Flow rate, solvent polarity, column length, temperature, and particle size of the adsorbent.
Q24. What are fractions in Column Chromatography?
Ans: Fractions are the separate portions of eluent collected at different times, each containing one or more purified compounds.
Q25. What is the importance of Column Chromatography in biology?
Ans: It is essential for purifying biomolecules like proteins, amino acids, nucleic acids, and enzymes, ensuring accurate biological and biochemical research.
References
- Wilson, K., Walker, J. (2018). Principles and Techniques of Biochemistry and Molecular Biology (8 eds.). Cambridge University Press: New York.
- https://microbenotes.com/column-chromatography/
- https://chromatography.conferenceseries.com/events-list/applications-of-chromatography
- https://www.slideshare.net/RameshJupudi/column-chromatography-ppt
- https://www.slideshare.net/krakeshguptha/column-chromatography-26966949
- https://en.wikipedia.org/wiki/Column_chromatography
Other related topics you might be interested in:
Chromatography – Principle, Types, Steps, Uses, and Advantages
Paper Chromatography – Definition, Principle, Types, Steps, Applications, Advantages & Limitations
Thin Layer Chromatography (TLC) – Principle, Steps, Applications, Advantages & Limitations
Gas Chromatography (GC) – Principle, Parts, Procedure, Steps, Applications, Advantages & Limitations
Ion Exchange Chromatography – Principle, Instrumentation, Procedure, Applications
High-Performance Liquid Chromatography (HPLC) – Principle, Instrumentation, Types & Applications
Adsorption Chromatography – Principle, Types, Procedure, Applications & Advantages
Affinity Chromatography – Principle, Components, Procedure, Applications & Advantages