Introduction to Gamma-ray Spectroscopy
- Gamma rays (γ-rays) are the highest-energy form of electromagnetic radiation.
- They are produced by radioactive decay of atomic nuclei, nuclear reactions, and cosmic events such as supernovae, black holes, and neutron stars.
- Because gamma rays are highly energetic, they can penetrate matter deeply, making them useful for studying nuclear properties, radioactive isotopes, and space phenomena.
Gamma-ray spectroscopy (GRS) is a technique that measures the energy distribution of gamma rays emitted by a radioactive sample. By analyzing this spectrum, scientists can identify and quantify specific isotopes present in the material.
In simple words: Gamma-ray spectroscopy is like a fingerprinting tool for radioactive elements.
Principle of Gamma-ray Spectroscopy
- Radioactive isotopes emit gamma rays at specific energy levels that are unique to each isotope.
- When these gamma rays strike a detector, they produce signals proportional to their energy.
- A spectrum is generated, showing peaks at energies corresponding to specific isotopes.
- By comparing the measured energies with reference data, the identity and quantity of isotopes can be determined.
Key Points:
- Each radioactive isotope has a unique gamma-ray spectrum.
- The intensity of peaks represents the abundance of that isotope.
- This allows both qualitative (identification) and quantitative (measurement) analysis.
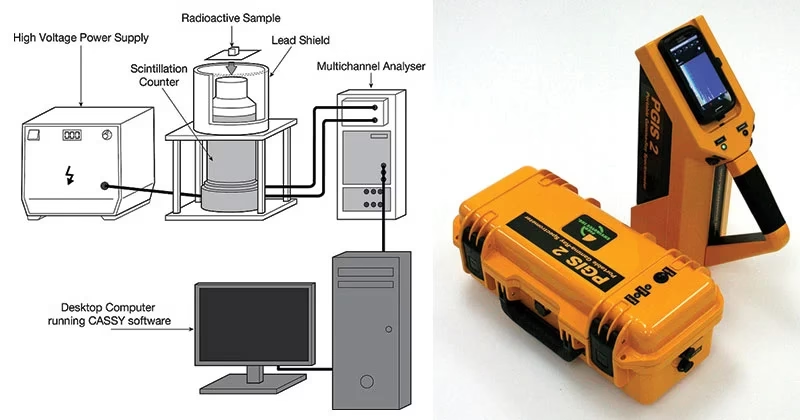
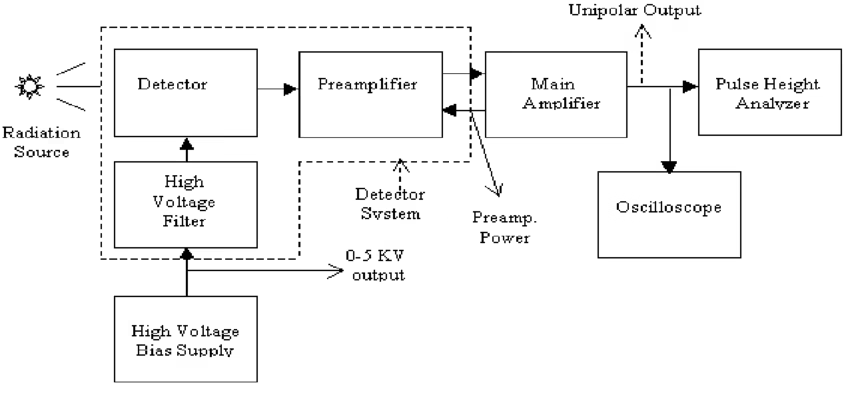
Components of Gamma-ray Spectroscopy
The basic components of a Gamma-ray Spectroscopy system include:
1. Radiation Detector
Detects gamma rays and converts them into measurable signals. Common types:
(a) Scintillation Detectors
- Work on the principle of scintillation (light emission when radiation hits a material).
- Sodium iodide (NaI) crystals doped with thallium [NaI(Tl)] are widely used.
- The crystal emits tiny flashes of light when struck by gamma rays.
- A photomultiplier tube (PMT) converts these light flashes into electrical signals.
(b) Semiconductor Detectors
- Use semiconductors like Germanium (Ge).
- Gamma rays excite electrons from valence band to conduction band, producing current.
- Provide high resolution compared to scintillation detectors.
- Common types: Ge(Li) detectors and High Purity Germanium (HPGe) detectors.
2. Electronics System
- Amplifies and processes signals from the detector.
- Components:
- Amplifiers – boost weak signals.
- Pulse Height Analyzers / Multichannel Analyzers (MCA) – separate signals based on energy.
- Data Storage & Display – converts signals into a readable spectrum.
3. Computer/Display Unit
- Generates the gamma-ray spectrum graph (intensity vs energy).
- Helps in analysis, identification, and quantification of isotopes.
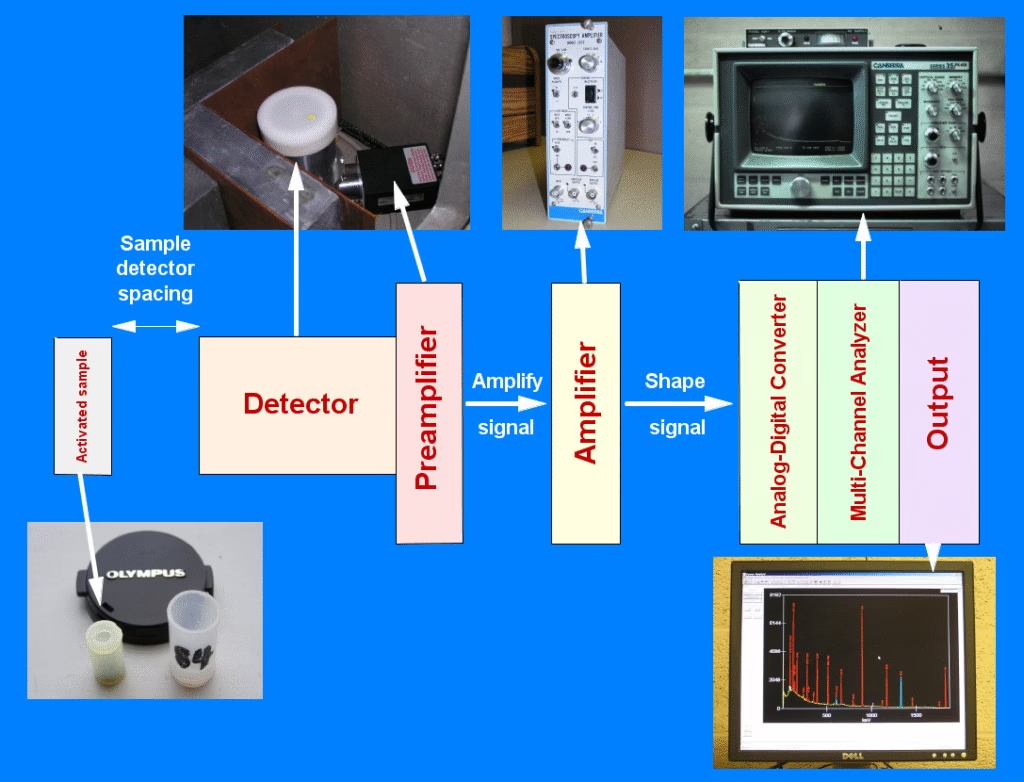
Working of Gamma-ray Spectroscopy
- Sample Exposure
- A radioactive sample emits gamma rays naturally.
- Detection
- Gamma rays enter the detector (NaI(Tl) crystal or HPGe detector).
- Signal Conversion
- Energy is converted into light (scintillation) or electrical charge (semiconductor).
- Signal Processing
- Signals are amplified and sorted based on their energy.
- Spectrum Generation
- A graph is produced showing peaks at specific energy levels.
- Analysis
- Peaks are compared with reference data to identify isotopes and their concentrations.
Applications of Gamma-ray Spectroscopy
1. Nuclear Physics & Research
- Study of nuclear structure and transitions.
- Analysis of nuclear reactions and decay processes.
2. Radiometric Assay
- Identifying and quantifying radioactive isotopes.
- Essential in nuclear medicine, power plants, and radiological safety.
3. Environmental Studies
- Detection of radioactive contamination in soil, water, and air.
- Monitoring fallout from nuclear accidents (e.g., Chernobyl, Fukushima).
4. Space and Planetary Research
- Onboard instruments in space missions (like lunar and Mars orbiters) use GRS to detect elements such as hydrogen, oxygen, and iron.
- Helps study planetary crust composition and search for water on extraterrestrial bodies.
5. Medical Applications
- Used in nuclear medicine imaging.
- Tracer isotopes (like I-131, Tc-99m) emit gamma rays that can be analyzed for diagnostic purposes.
6. Industrial Applications
- Quality control in steel and cement industries.
- Detecting isotopes in radioactive waste management.
- Used in oil and gas exploration to analyze geological formations.
Advantages of Gamma-ray Spectroscopy
- Non-destructive method of analysis.
- High sensitivity – detects even trace amounts of isotopes.
- Quick and reliable identification of isotopes.
- Provides both qualitative and quantitative information.
- Can be applied in nuclear, environmental, medical, and space sciences.
Limitations of Gamma-ray Spectroscopy
- Requires special handling and safety precautions due to radiation.
- Expensive detectors (especially HPGe) that need liquid nitrogen cooling.
- Limited resolution in scintillation detectors compared to semiconductor detectors.
- Only applicable to radioactive samples (cannot analyze stable materials).
Conclusion
- Gamma-ray Spectroscopy is a key tool in modern science for analyzing radioactive isotopes and understanding nuclear processes.
- It works on the principle that each isotope emits unique gamma-ray energies, which can be detected and analyzed.
- With applications in nuclear physics, medicine, environmental science, industry, and space exploration, it is one of the most versatile spectroscopy methods.
- Despite limitations like high cost and radiation safety issues, its accuracy, sensitivity, and non-destructive nature make it indispensable.
In short: Gamma-ray Spectroscopy is a window into the atomic nucleus, helping us unlock secrets of matter, energy, and the universe.
Frequently Asked Questions (FAQs) on Gamma-ray Spectroscopy
Q1. What is Gamma-ray Spectroscopy?
Ans: Gamma-ray Spectroscopy is an analytical technique that measures the energy spectrum of gamma rays emitted by radioactive isotopes to identify and quantify them.
Q2. What is the principle of Gamma-ray Spectroscopy?
Ans: Each radioactive isotope emits gamma rays at unique energy levels. By detecting and analyzing these energy peaks, isotopes can be identified and measured.
Q3. What types of detectors are used in Gamma-ray Spectroscopy?
Ans: The two most common detectors are:
- Scintillation detectors – usually NaI(Tl) crystals.
- Semiconductor detectors – like High Purity Germanium (HPGe).
Q4. Which detector gives better resolution – NaI(Tl) or HPGe?
Ans: HPGe detectors provide much higher energy resolution than NaI(Tl), making them more precise but also more expensive and requiring cooling with liquid nitrogen.
Q5. What are the main components of a Gamma-ray Spectroscopy system?
Ans:
- Detector (scintillation or semiconductor).
- Amplifier and electronics.
- Multichannel analyzer (MCA).
- Computer/display unit for spectrum analysis.
Q6. What is a gamma-ray spectrum?
Ans: A gamma-ray spectrum is a graph of counts (intensity) vs energy, where each peak corresponds to a radioactive isotope.
Q7. What are the applications of Gamma-ray Spectroscopy?
Ans:
- Nuclear physics – studying nuclear structure and decay.
- Medicine – nuclear imaging and tracer studies.
- Environmental monitoring – detecting radioactive contamination.
- Space research – planetary surface composition.
- Industry – material testing and waste management.
Q8. How is Gamma-ray Spectroscopy used in space exploration?
Ans: Space probes carry gamma-ray spectrometers to study planetary crust composition, detect elements like hydrogen and iron, and search for water on other planets.
Q9. How is Gamma-ray Spectroscopy used in medicine?
Ans: It is used in nuclear medicine to detect gamma emissions from tracer isotopes like Technetium-99m or Iodine-131 for diagnosis and imaging.
Q10. Is Gamma-ray Spectroscopy non-destructive?
Ans: Yes. The technique is non-destructive, meaning the sample remains intact during analysis.
Q11. What are the advantages of Gamma-ray Spectroscopy?
Ans:
- High sensitivity and accuracy.
- Non-destructive method.
- Provides both qualitative and quantitative data.
- Wide applications across multiple sciences.
Q12. What are the limitations of Gamma-ray Spectroscopy?
Ans:
- High cost of detectors (especially HPGe).
- Requires skilled operators.
- Radiation safety precautions are essential.
- Cannot analyze stable, non-radioactive materials.
Q13. What is the role of a scintillation detector in Gamma-ray Spectroscopy?
Ans: A scintillation detector converts gamma rays into tiny flashes of light (scintillations), which are then converted into electrical signals by a photomultiplier tube.
Q14. Why is liquid nitrogen needed for HPGe detectors?
Ans: HPGe detectors require cooling with liquid nitrogen to reduce electronic noise and improve resolution.
Q15. Can Gamma-ray Spectroscopy detect trace elements?
Ans: Yes. It is highly sensitive and can detect trace levels of radioactive isotopes.
Q16. What is the difference between Gamma-ray Spectroscopy and X-ray Spectroscopy?
Ans:
- Gamma-ray Spectroscopy – deals with high-energy photons emitted from nuclear transitions.
- X-ray Spectroscopy – deals with lower-energy photons from electronic transitions.
Q17. How does Gamma-ray Spectroscopy help in environmental studies?
Ans: It is used to monitor radioactive pollution in soil, water, and air, and to study fallout from nuclear accidents.
Q18. What is radiometric assay in Gamma-ray Spectroscopy?
Ans: It refers to the identification and quantification of radioactive isotopes using gamma-ray analysis.
Q19. Can portable Gamma-ray Spectrometers be used in the field?
Ans: Yes. Portable devices are available and are widely used in environmental monitoring, mining, and security screening.
Q20. What safety precautions are required in Gamma-ray Spectroscopy?
Ans: Proper shielding, radiation monitoring, protective gear, and strict handling procedures must be followed due to the ionizing nature of gamma rays.
Q21. Is Gamma-ray Spectroscopy qualitative or quantitative?
Ans: It is both:
- Qualitative – identifies which isotopes are present.
- Quantitative – measures the concentration of each isotope.
Q22. Why is Gamma-ray Spectroscopy important in nuclear science?
Ans: Because it provides detailed insights into nuclear decay, isotope distribution, and nuclear structure, making it indispensable in nuclear research and safety monitoring.
References
- https://en.wikipedia.org/wiki/Gamma_spectroscopy
- https://microbenotes.com/gamma-ray-%ce%b3-ray-spectroscopy/
- https://archive.cnx.org/contents/686b9c8b-1656-49ec-a969-84da62a60eca@1/principles-of-gamma-ray-spectroscopy-and-applications-in-nuclear-forensics
- https://owlcation.com/stem/Gamma-Ray-Spectroscopy
Other related topics you might be interested in:
Mass Spectrometry – Principle, Steps, Instrumentation, Types & Applications
Spectrophotometer – Principle, Components, Applications, Advantages & Limitations
Types of Spectroscopy – Principles, Types, Steps, and Applications
UV-Vis Spectroscopy – Principle, Instrumentation, Applications, Advantages & Limitations