What is Infrared (IR) Spectroscopy?
- Infrared (IR) Spectroscopy, also called Vibrational Spectroscopy, is an analytical technique that studies the interaction of infrared radiation with matter.
- It is based on the fact that molecules absorb IR light at frequencies that match their natural vibrational modes.
- When a compound absorbs IR radiation, specific chemical bonds stretch, bend, or vibrate.
- The recorded data is displayed as an infrared spectrum, which is unique for every compound (like a fingerprint).
- Because of its simplicity, reliability, and broad applicability, IR spectroscopy is one of the most commonly used spectroscopic tools in chemistry, biology, material science, food science, and forensic analysis.
In short: Infrared spectroscopy helps identify molecules by detecting their vibrations, just like recognizing people by their fingerprints.

Principle of Infrared (IR) Spectroscopy
- IR spectroscopy is based on the absorption of infrared radiation by molecules.
- Each molecule absorbs IR light of a certain frequency depending on the type of bond and the atoms involved.
- The absorbed energy causes vibrational excitation (stretching, bending, twisting).
- The IR spectrum is obtained as a graph of absorbance (or transmittance) vs. frequency/wavenumber.
Key Points:
- Wavelength range: 2500–16000 nm
- Frequency range: 1.9 × 10¹³ – 1.2 × 10¹⁴ Hz
- Photon energy: 1–15 kcal/mol (enough for vibrations but not for electronic excitation).
- Bond properties: Stronger bonds and lighter atoms vibrate at higher frequencies.
- Outcome: The spectrum gives information about functional groups and molecular structure.
That is why IR spectroscopy is called a functional group analyzer in chemistry.
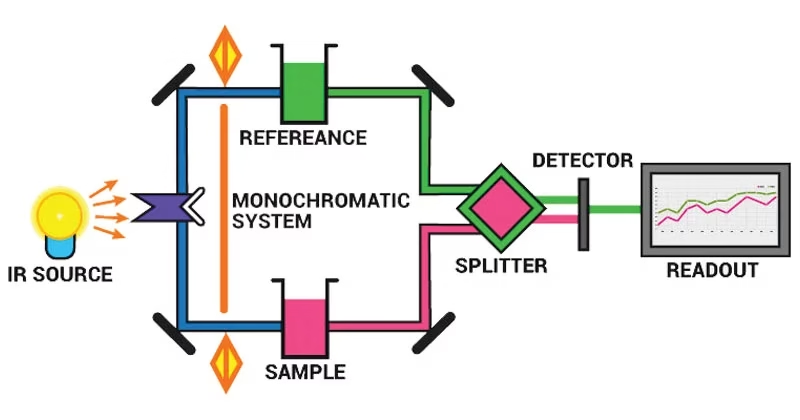
Instrumentation of Infrared (IR) Spectroscopy
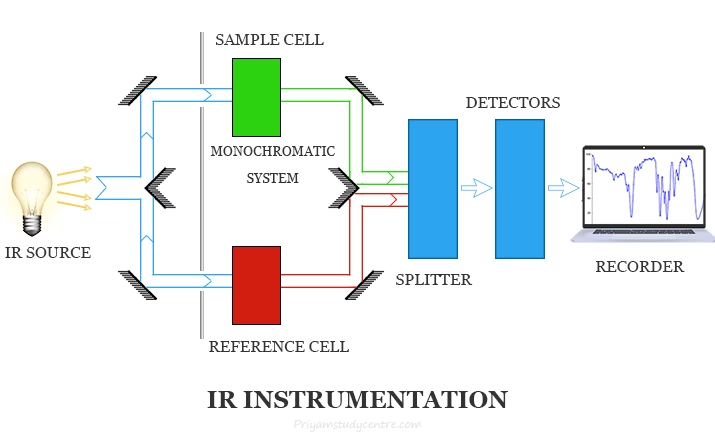
The IR spectrometer is the instrument used to perform IR spectroscopy. It consists of several essential parts:
1. Radiation Source
- Provides steady IR radiation covering the required wavelength range.
- Common sources:
- Nernst Glower (rare-earth oxides heated electrically)
- Globar Source (silicon carbide rod)
- Incandescent lamp
- Tungsten lamp
- Mercury arc
- Nichrome wire
2. Sample Handling & Sample Cells
- IR can analyze solid, liquid, and gas samples.
- Special alkali halide cells (NaCl, KBr) are used because glass absorbs IR.
- Solids: made into KBr pellets or suspended as Nujol mulls.
- Liquids: placed between polished salt plates.
- Gases: studied in long path-length gas cells.
3. Monochromator
- Separates IR radiation into different wavelengths.
- Types:
- Prisms (KBr, NaCl, CsI)
- Diffraction gratings (alkali halides)
- Filters (lithium fluoride filters)
4. Detector
- Measures the intensity of transmitted IR radiation.
- Common detectors:
- Thermocouples
- Bolometers
- Thermistors
- Golay cell
- Pyroelectric detectors
5. Recorder / Computer System
- Converts detector signals into an infrared spectrum.
- Modern IR spectrometers use Fourier Transform Infrared (FTIR) technology for faster and more accurate results.
Types of Infrared (IR) Spectroscopy
- Near-IR Spectroscopy (14000–4000 cm⁻¹):
- Analyzes overtone and combination vibrations.
- Used in food, pharmaceutical, and agricultural studies.
- Mid-IR Spectroscopy (4000–400 cm⁻¹):
- Most commonly used.
- Provides detailed information about functional groups.
- Far-IR Spectroscopy (400–10 cm⁻¹):
- Useful for studying lattice vibrations, heavy atom bonds, and inorganic compounds.
Applications of Infrared (IR) Spectroscopy
Infrared spectroscopy is extremely versatile. Its main uses include:
- Identification of Compounds – IR acts as a fingerprinting tool.
- Protein & Enzyme Studies – analyzing secondary structures (α-helix, β-sheets).
- Nanoscale Semiconductor Analysis – useful in material sciences.
- Space Exploration – used in telescopes to identify chemical composition of planets.
- Environmental Studies – detection of pollutants and greenhouse gases.
- Food Industry – checking adulteration and quality control.
- Pharmaceuticals – drug purity testing and formulation studies.
- Forensic Science – identification of inks, drugs, explosives.
Advantages of Infrared (IR) Spectroscopy
- Non-destructive method.
- Requires small sample quantity.
- Works with solids, liquids, and gases.
- Provides unique molecular fingerprint.
- Fast and reliable.
- Can detect impurities.
Conclusion
- Infrared (IR) Spectroscopy is one of the most powerful and widely used analytical tools.
- It provides fast, reliable, and non-destructive analysis of molecules.
- With applications ranging from protein studies to forensic investigations, IR spectroscopy remains an essential method in modern laboratories.
In simple terms: IR spectroscopy allows scientists to “listen” to molecular vibrations to understand what a molecule is made of.
Frequently Asked Questions (FAQs) on Infrared (IR) Spectroscopy
Q1. What is Infrared (IR) Spectroscopy?
Ans: Infrared Spectroscopy is an analytical technique that studies how molecules absorb infrared radiation and undergo vibrational transitions. It is mainly used to identify functional groups and analyze molecular structures.
Q2. What is the principle of IR Spectroscopy?
Ans: It works on the principle that molecules absorb specific frequencies of IR radiation, which match their natural vibrational frequencies. These absorbed frequencies are recorded as peaks in an IR spectrum.
Q3. Why is IR Spectroscopy called a fingerprint technique?
Ans: Every compound produces a unique IR spectrum, just like a human fingerprint, allowing scientists to identify unknown compounds with high accuracy.
Q4. What is the useful range of IR radiation for spectroscopy?
Ans: The IR region useful for analysis is 2,500–16,000 nm (wavenumber range: 4000–625 cm⁻¹).
Q5. What are the three main regions of IR spectroscopy?
Ans:
- Near IR: 14,000–4000 cm⁻¹
- Mid IR: 4000–400 cm⁻¹ (most useful for functional group analysis)
- Far IR: 400–10 cm⁻¹
Q6. What are the main applications of IR Spectroscopy?
Ans: Used in organic chemistry, pharmaceuticals, protein studies, food testing, forensic science, environmental monitoring, and space research.
Q7. What are common sample preparation methods in IR spectroscopy?
Ans:
- KBr pellets for solids
- Nujol mulls (suspension in mineral oil)
- Liquid films between salt plates
- Gas cells for gaseous samples
Q8. Why is KBr used in IR spectroscopy?
Ans: Because KBr is transparent to IR radiation, it does not interfere with absorption and allows accurate spectra recording.
Q9. What is FTIR (Fourier Transform Infrared) Spectroscopy?
Ans: FTIR is a modern IR technique that uses mathematical Fourier Transform to produce high-resolution, accurate, and fast IR spectra.
Q10. What are common detectors in IR spectrometers?
Ans: Thermocouples, bolometers, Golay cells, and pyroelectric detectors.
Q11. What is the difference between Near IR and Mid IR spectroscopy?
Ans:
- Near IR: Studies overtones and combination bands, useful in food and pharma.
- Mid IR: Provides functional group information, most widely used.
Q12. Can IR spectroscopy detect impurities?
Ans: Yes. Extra peaks or unusual absorptions in the IR spectrum indicate the presence of impurities.
Q13. What are the advantages of IR spectroscopy?
Ans:
- Non-destructive technique
- Requires small sample size
- Provides unique molecular fingerprint
- Works for solids, liquids, and gases
- Fast and reliable
Q14. What are the limitations of IR spectroscopy?
Ans:
- Cannot provide complete molecular structure
- Requires pure and dry samples
- Quantitative analysis is limited
- Similar compounds may give overlapping spectra
Q15. How is IR spectroscopy used in biology?
Ans: Used to study proteins, enzymes, nucleic acids, and biomolecular interactions.
Q16. How is IR spectroscopy used in the pharmaceutical industry?
Ans: For drug identification, purity testing, and quality control.
Q17. What is the role of IR spectroscopy in forensic science?
Ans: It is used to identify drugs, inks, poisons, toxins, and explosives in crime investigations.
Q18. What solvents are suitable for IR spectroscopy?
Ans: Solvents that do not absorb in the IR region such as CCl₄ (carbon tetrachloride) and CS₂ (carbon disulfide).
Q19. What is an absorption band in IR spectroscopy?
Ans: A peak in the IR spectrum that represents the absorption of radiation by a specific bond vibration.
Q20. What is the difference between IR and UV spectroscopy?
Ans:
- IR spectroscopy: Studies vibrational transitions in molecules.
- UV spectroscopy: Studies electronic transitions in molecules.
Q21. Can IR spectroscopy be used for quantitative analysis?
Ans: While mainly qualitative, it can be used semi-quantitatively by comparing band intensities.
Q22. Why is IR spectroscopy important in environmental studies?
Ans: It helps detect pollutants, greenhouse gases, and toxic chemicals in air and water samples.
References
- https://en.wikipedia.org/wiki/Infrared_spectroscopy
- https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/spectrpy/infrared/infrared.html
- http://www.wag.caltech.edu/home/jang/genchem/infrared.html
- https://www.pharmatutor.org/pharma-analysis/analytical-aspects-of-infra-red spectroscopy-ir/instrumentation-of-ir-spectrophotometry
- https://microbenotes.com/infrared-ir-spectroscopy/
Other related topics you might be interested in:
Mass Spectrometry – Principle, Steps, Instrumentation, Types & Applications
Spectrophotometer – Principle, Components, Applications, Advantages & Limitations
Types of Spectroscopy – Principles, Types, Steps, and Applications
UV-Vis Spectroscopy – Principle, Instrumentation, Applications, Advantages & Limitations