Introduction to NMR Spectroscopy
- NMR Spectroscopy (Nuclear Magnetic Resonance Spectroscopy) is one of the most advanced and widely used analytical techniques in modern chemistry and biology.
- It is based on the principle that certain nuclei behave like tiny magnets when placed in a magnetic field and can absorb radiofrequency (RF) radiation, leading to measurable resonance signals.
- NMR is widely applied in structural determination, purity testing, drug design, biomolecular studies, and clinical imaging (MRI).
In simple words: NMR spectroscopy is like an atomic-level camera that helps us see the structure, bonding, and dynamics of molecules.

Principle of NMR Spectroscopy
- Many nuclei possess spin (a type of angular momentum).
- Nuclei with spin behave like tiny charged magnets.
- When placed in an external magnetic field (B₀), these nuclei align either with or against the field, creating two distinct energy levels.
- When radiofrequency radiation is applied, nuclei absorb energy and transition from the lower to the higher energy state.
- As they relax back, they release signals, which are recorded as an NMR spectrum.
Key Concepts:
- Resonance: The process where nuclei absorb RF radiation and move between energy states.
- Chemical Shift (δ): A measure of how shielded/deshielded a nucleus is by its electronic environment.
- Spin–spin coupling: Interaction between nuclear spins gives detailed splitting patterns.
This makes NMR unique in providing detailed structural, dynamic, and electronic information about molecules.
Working of NMR Spectroscopy
- Sample Placement: The sample is dissolved in a suitable solvent (often deuterated solvents like CDCl₃, D₂O).
- Magnetic Field Application: The nuclei align with or against the external magnetic field.
- Radiofrequency Pulse: A short RF pulse excites the nuclei.
- Relaxation & Signal Emission: Nuclei return to their lower energy states, releasing RF signals.
- Detection: These signals are captured and processed to generate a spectrum.
- Spectrum Analysis: Peaks and their positions provide structural and quantitative information.
Instrumentation of NMR Spectroscopy
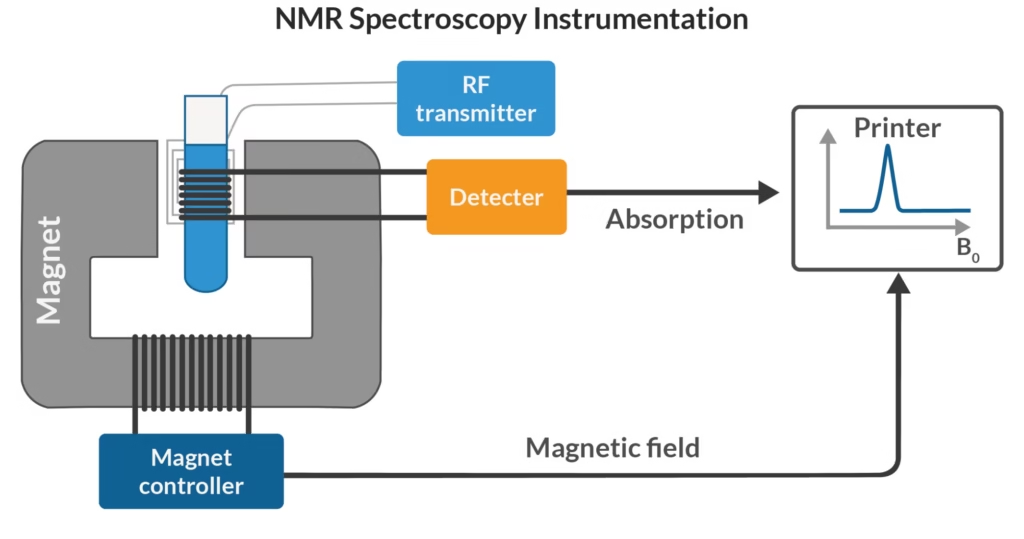
1. Sample Holder
- Usually a thin glass tube (8.5 cm long, 0.3 cm in diameter).
- Must be transparent to RF radiation.
2. Magnet
- Provides a strong and uniform magnetic field (60–100 MHz in classical NMR; modern superconducting magnets go up to 1 GHz).
- Can be permanent, electromagnets, or superconducting magnets.
3. Magnetic Coils
- Generate auxiliary fields for calibration and field homogeneity.
4. Sweep Generator
- Produces controlled variations in the applied magnetic field to detect resonance conditions.
5. Radiofrequency Transmitter
- Delivers RF pulses that excite nuclei.
6. Receiver Coil
- Detects emitted signals when nuclei relax.
7. Computer/Readout System
- Converts raw signals into spectra and analyzes chemical shifts, splitting, and intensities.
Types of NMR Spectroscopy
- ¹H-NMR (Proton NMR):
- Most common, provides detailed information about hydrogen atoms in organic molecules.
- ¹³C-NMR (Carbon-13 NMR):
- Provides structural information about carbon skeletons.
- ²D-NMR (Two-Dimensional NMR):
- Advanced method for studying interactions between nuclei (COSY, NOESY, HSQC).
- Solid-State NMR:
- For insoluble samples, polymers, and crystalline solids.
- Time-domain NMR:
- Used to study molecular dynamics in solution.
Steps in NMR Spectroscopy
- Sample Preparation – Dissolve in deuterated solvent (CDCl₃, D₂O).
- Placement in Magnetic Field – Aligns nuclear spins.
- Excitation with RF Radiation – Nuclei absorb energy.
- Relaxation & Signal Detection – Emitted RF captured.
- Spectrum Recording – Data transformed into spectrum.
- Interpretation – Chemical shifts, coupling constants, and peak intensities analyzed.
Applications of NMR Spectroscopy
- Structural Elucidation of organic and inorganic compounds.
- Protein and Nucleic Acid Studies (secondary and tertiary structures).
- Drug Discovery & Pharmaceuticals – checking drug purity and molecular interaction.
- Metabolomics – analysis of metabolic profiles in biological samples.
- Forensic Science – chemical identification in crime evidence.
- Food Industry – detecting adulterants and ensuring quality.
- Medical Imaging (MRI): Based on NMR principle.
Advantages of NMR Spectroscopy
- Provides complete structural information.
- Non-destructive to the sample.
- Applicable to liquids, solids, and biomolecules.
- Quantitative as well as qualitative.
- Used for dynamic and kinetic studies.
- Replaces X-ray crystallography for some protein structures.
Limitations of NMR Spectroscopy
- Very expensive instruments (superconducting magnets).
- Requires high sample concentration.
- Limited sensitivity for nuclei with low natural abundance (e.g., ¹³C, ¹⁵N).
- Sample preparation (especially deuterated solvents) is costly.
- Data interpretation can be complex.
Conclusion
- NMR Spectroscopy is one of the most powerful tools for understanding the molecular world.
- It provides structural, dynamic, and chemical information that no other spectroscopy method can fully match.
- With applications ranging from organic chemistry to medicine, it remains a gold standard technique despite its cost and complexity.
In summary: NMR spectroscopy is a molecular detective tool, revealing the hidden details of atomic arrangements and interactions.
Frequently Asked Questions (FAQs) on NMR Spectroscopy
Q1. What is NMR Spectroscopy?
Ans: NMR (Nuclear Magnetic Resonance) Spectroscopy is an analytical technique that studies how certain nuclei behave in a magnetic field when exposed to radiofrequency (RF) radiation. It helps determine the structure, purity, and dynamics of molecules.
Q2. Who discovered NMR Spectroscopy?
Ans: NMR was independently discovered in 1946 by Felix Bloch and Edward Purcell, who later received the Nobel Prize in Physics (1952).
Q3. What is the principle of NMR Spectroscopy?
Ans: Nuclei with spin absorb RF energy in the presence of an external magnetic field and undergo transitions between energy levels. This absorption produces signals that are converted into an NMR spectrum.
Q4. Which nuclei are commonly studied in NMR?
Ans: The most widely studied are ¹H (proton NMR) and ¹³C (carbon NMR). Other useful nuclei include ¹⁹F, ³¹P, ²³Na, ¹⁵N.
Q5. What is a chemical shift in NMR?
Ans: Chemical shift (δ) is the difference in resonance frequency of a nucleus relative to a reference standard (usually TMS – Tetramethylsilane) expressed in parts per million (ppm).
Q6. Why is TMS used in NMR Spectroscopy?
Ans: TMS is chemically inert, volatile, and produces a single sharp peak at 0 ppm, making it an ideal reference standard.
Q7. What is spin-spin coupling in NMR?
Ans: It refers to the splitting of NMR signals into multiplets due to the interaction between neighboring nuclear spins.
Q8. What are coupling constants (J values)?
Ans: The distance between split peaks (measured in Hertz, Hz) in a multiplet, providing information about neighboring atoms.
Q9. What solvents are used in NMR Spectroscopy?
Ans: Deuterated solvents like CDCl₃, D₂O, DMSO-d₆, and CD₃OD are used because they don’t interfere with the proton signals.
Q10. What is ¹H NMR (Proton NMR)?
Ans: A type of NMR that studies hydrogen atoms in molecules, providing information on number of protons, their environment, and connectivity.
Q11. What is ¹³C NMR?
Ans: A method that analyzes carbon atoms in molecules, useful for studying the carbon framework of organic compounds.
Q12. What is 2D NMR Spectroscopy?
Ans: Two-dimensional NMR (COSY, NOESY, HSQC, HMBC) gives correlation between nuclei, providing detailed structural and spatial information.
Q13. What is solid-state NMR?
Ans: NMR technique used for insoluble samples, polymers, membranes, and crystalline solids, unlike solution NMR.
Q14. What are relaxation times in NMR?
Ans:
- T₁ (Spin-lattice relaxation): Time taken for nuclei to release energy to surroundings.
- T₂ (Spin-spin relaxation): Time taken for spins to lose coherence among themselves.
Q15. What is FT-NMR (Fourier Transform NMR)?
Ans: A modern technique where signals are collected in time domain and then converted to frequency domain using Fourier Transform, giving high sensitivity and resolution.
Q16. How is NMR used in drug discovery?
Ans: It helps in studying drug–receptor interactions, structural confirmation, purity testing, and metabolic pathways.
Q17. How is NMR different from IR or UV Spectroscopy?
Ans:
- NMR: Provides detailed atomic-level structure.
- IR: Identifies functional groups.
- UV: Studies electronic transitions and conjugation.
Q18. What are the advantages of NMR Spectroscopy?
Ans:
- Non-destructive
- Provides complete structural information
- Applicable to solids and liquids
- Useful in quantitative as well as qualitative analysis
Q19. What are the limitations of NMR Spectroscopy?
Ans:
- Very expensive instruments
- Requires high sample concentration
- Data interpretation is complex
- Low sensitivity for nuclei with low natural abundance
Q20. How is NMR used in biology?
Ans: NMR helps in studying proteins, nucleic acids, metabolites, and biomolecular interactions, and is widely used in metabolomics and structural biology.
Q21. How is NMR used in medicine?
Ans: The principle of NMR is applied in Magnetic Resonance Imaging (MRI) for non-invasive medical diagnostics.
Q22. Is NMR spectroscopy quantitative?
Ans: Yes. NMR is both qualitative and quantitative. The integrals of NMR signals are directly proportional to the number of nuclei present.
References
- https://microbenotes.com/nuclear-magnetic-resonance-nmr-spectroscopy/
- https://www.slideshare.net/solairajananant/nmr-spectroscopy-13887430
- https://www.ias.ac.in/article/fulltext/reso/009/01/0034-0049
- https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/spectrpy/nmr/nmr1.htm
- https://en.wikipedia.org/wiki/Nuclear_magnetic_resonance_spectroscopy
Other related topics you might be interested in:
Mass Spectrometry – Principle, Steps, Instrumentation, Types & Applications
Spectrophotometer – Principle, Components, Applications, Advantages & Limitations
Types of Spectroscopy – Principles, Types, Steps, and Applications
UV-Vis Spectroscopy – Principle, Instrumentation, Applications, Advantages & Limitations
