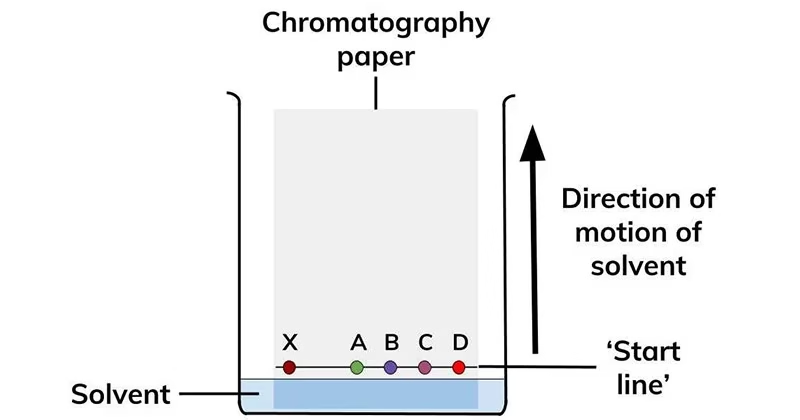
Introduction to Paper Chromatography
- Chromatography is a separation technique used to isolate components of a mixture based on their different affinities toward a stationary phase and a mobile phase.
- Paper Chromatography (PC) is a form of planar chromatography where the separation takes place on a specialized filter paper.
- It is one of the earliest and simplest forms of chromatography, widely used in chemical, biological, environmental, and pharmaceutical studies.
- The technique was first introduced by the German scientist Christian Friedrich Schönbein in 1865, and later refined into a powerful analytical method.
- Paper chromatography is especially popular in teaching laboratories due to its simplicity, low cost, and effectiveness in demonstrating basic principles of separation.
In short: Paper chromatography is a low-cost, easy-to-use, yet powerful separation technique for both organic and inorganic compounds.
Principle of Paper Chromatography
- Separation in paper chromatography is based mainly on partition principle.
- The stationary phase is the water or moisture held in the cellulose fibers of the filter paper.
- The mobile phase is a suitable organic solvent or buffer that moves through the paper by capillary action.
- Different compounds in the sample have different solubility in the mobile phase and different affinity toward the stationary phase.
- Those with higher solubility in the solvent travel faster, while those that bind strongly to the stationary phase move slowly.
- This differential migration leads to the separation of components.
Each substance has a characteristic Rf value (retardation factor), which helps in identification.
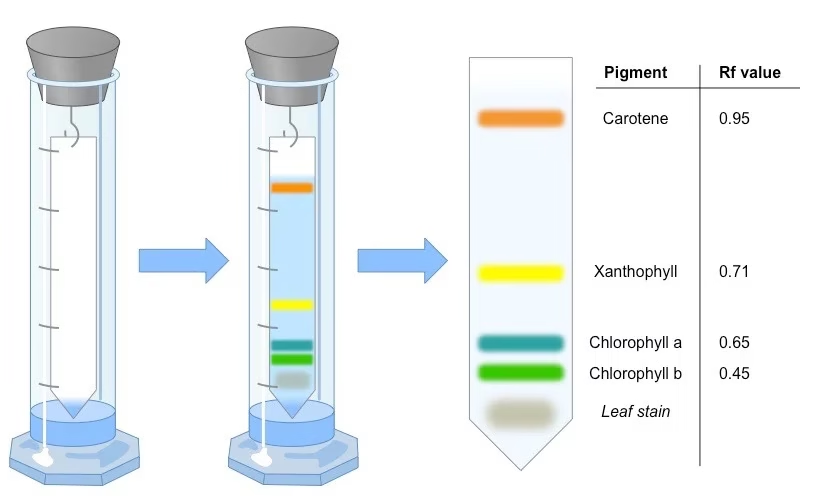
Types of Paper Chromatography
1. Paper Adsorption Chromatography
- Paper is impregnated with adsorbents like silica or alumina.
- Separation occurs mainly due to adsorption interactions between compounds and the stationary phase.
2. Paper Partition Chromatography
- The stationary phase is the moisture/water in cellulose fibers of filter paper.
- The mobile phase is an organic solvent.
- This is the most common form of paper chromatography.
3. Ascending Chromatography
- The solvent moves upward against gravity.
- Commonly used for amino acid and plant pigment separation.
4. Descending Chromatography
- The solvent reservoir is at the top, and the solvent flows downward.
- Faster separation due to gravity-assisted flow.
5. Ascending–Descending Chromatography
- A hybrid method: solvent first moves upward and then downward, allowing longer separation path.
6. Circular / Radial Chromatography
- The sample is placed at the center of circular paper.
- Solvent spreads outward radially, giving circular spots.
Components of Paper Chromatography
Paper chromatography requires simple and inexpensive equipment:
1. Stationary Phase (Paper)
- Whatman filter papers (No.1, No.2, No.3, etc.) are commonly used.
- Paper composition: 98–99% α-cellulose and 0.3–1% β-cellulose.
- Modified papers:
- Acid/base washed paper
- Glass fiber papers
- Hydrophilic papers (modified with methanol, glycerol, glycol)
- Hydrophobic papers (acetylated OH groups for reverse-phase chromatography)
- Papers impregnated with silica, alumina, or ion-exchange resins
2. Mobile Phase (Solvents)
- Choice depends on the nature of analyte.
- Polar solvents: methanol, ethanol, water, butanol, acetic acid.
- Non-polar solvents: kerosene, cyclohexane, dimethyl ether.
- Examples:
- Isopropanol : Ammonia : Water (9:1:2)
- Methanol : Water (4:1)
- Butanol : Acetic acid : Water (12:3:5)
3. Developing Chamber
- A glass or plastic tank that holds the solvent and paper.
- Must be saturated with solvent vapor to ensure uniform movement.
4. Visualization Reagents
- Since many compounds are colorless, visualization is essential.
- Ninhydrin – detects amino acids.
- Iodine vapors – detect alkaloids, oils.
- UV light – for fluorescent compounds.
Steps in Paper Chromatography
- Selection of Paper – Choose paper with suitable porosity and resolution.
- Selection of Solvent (Mobile Phase) – Chosen based on polarity of analytes.
- Preparation of Tank – Inner wall is lined with filter paper and saturated with solvent vapor.
- Sample Preparation and Loading –
- Dissolve sample in a suitable solvent.
- Spot 2–20 μL of solution on paper using a micropipette.
- Dry the spot to prevent spreading.
- Development of Chromatogram – Place paper in chamber with solvent.
- Ascending / Descending / Circular techniques may be used.
- Drying the Paper – Mark solvent front, dry paper in oven or cabinet.
- Visualization – Spray with reagents or observe under UV light.
- Calculation of Rf Values –


Applications of Paper Chromatography
- Pharmaceuticals: Purity testing of drugs and formulations.
- Food Industry: Detecting contaminants, preservatives, and adulterants.
- Forensic Science: Identifying drugs, inks, and toxins.
- Biochemistry: Separation of amino acids, nucleotides, sugars.
- Plant Studies: Analyzing pigments during photosynthesis.
- Fermentation & Ripening Studies: Tracking biochemical changes.
- Cosmetics: Quality testing and detection of harmful chemicals.
Advantages of Paper Chromatography
- Simple, rapid, and inexpensive.
- Requires very small sample amounts.
- Works for both organic and inorganic compounds.
- Does not require expensive instruments.
- Can be used for teaching and research.
- Excellent resolving power for simple mixtures.
Limitations of Paper Chromatography
- Cannot handle large sample volumes.
- Not suitable for highly complex mixtures.
- Less accurate compared to modern methods like HPLC or GC.
- Quantitative analysis is limited.
- Requires careful solvent selection.
Conclusion
- Paper Chromatography is one of the simplest and most versatile separation methods, still widely used in labs, industries, and research.
- Its principle lies in the different partitioning of compounds between paper (stationary phase) and solvent (mobile phase).
- Despite limitations compared to advanced techniques like HPLC, it remains important for educational, analytical, and quick testing purposes.
Frequently Asked Questions (FAQs) on Paper Chromatography
Q1. What is paper chromatography?
Ans: Paper chromatography is a separation technique where filter paper acts as the stationary phase and a suitable solvent acts as the mobile phase to separate mixtures into their components.
Q2. What is the principle of paper chromatography?
Ans: It works on the partition principle – compounds distribute differently between the stationary phase (water in cellulose fibers) and the mobile phase (solvent).
Q3. Who discovered paper chromatography?
Ans: Paper chromatography was introduced by Christian Friedrich Schönbein in 1865, and later refined into a practical method.
Q4. What is the stationary phase in paper chromatography?
Ans: The water molecules bound in cellulose fibers of filter paper act as the stationary phase.
Q5. What is the mobile phase in paper chromatography?
Ans: The solvent or solvent mixture that moves by capillary action through the paper (e.g., butanol, ethanol, water, acetic acid).
Q6. What are the types of paper chromatography?
Ans:
- Adsorption chromatography
- Partition chromatography
- Ascending chromatography
- Descending chromatography
- Ascending–Descending chromatography
- Circular/Radial chromatography
Q7. Why is paper chromatography important?
Ans: It is cheap, simple, and effective for separating and identifying compounds, especially in teaching, food testing, and forensic labs.
Q8. What solvents are used in paper chromatography?
Ans: Common solvents include butanol, acetic acid, ethanol, methanol, ammonia solution, water, and hexane, depending on the analyte.
Q9. What are the visualization techniques in paper chromatography?
Ans:
- Ninhydrin spray – for amino acids
- Iodine vapors – for lipids, alkaloids
- UV light – for fluorescent compounds
Q10. What are the applications of paper chromatography?
Ans: Used in drug testing, food quality control, pigment analysis, forensic science, biochemical research, and cosmetic testing.
Q11. Can paper chromatography detect impurities?
Ans: Yes. Presence of extra spots compared to a pure standard indicates impurities.
Q12. Is paper chromatography qualitative or quantitative?
Ans: Mainly qualitative (identification), but can provide semi-quantitative data using Rf values.
Q13. What are the advantages of paper chromatography?
Ans:
- Simple and inexpensive
- Requires small sample size
- Works for many organic and inorganic compounds
- Non-destructive in many cases
Q14. What are the limitations of paper chromatography?
Ans:
- Not suitable for complex mixtures
- Less accurate than HPLC/GC
- Cannot handle large sample sizes
- Quantitative analysis is limited
Q15. Why is filter paper used in paper chromatography?
Ans: Because cellulose fibers in filter paper can hold water molecules, which act as the stationary phase.
Q16. What is ascending chromatography?
Ans: A type where the solvent moves upward against gravity.
Q17. What is descending chromatography?
Ans: A type where the solvent moves downward with gravity, allowing faster separation.
Q18. What is circular chromatography?
Ans: In this method, the sample is spotted at the center of circular paper and solvent spreads radially.
Q19. How is paper chromatography used in biology?
Ans: It helps separate amino acids, nucleotides, sugars, and plant pigments.
Q20. How is paper chromatography used in forensics?
Ans: To identify drugs, inks, toxins, and poisons in crime investigations.
Q21. Can paper chromatography be used in environmental studies?
Ans: Yes. It is used to detect pollutants, pesticides, and chemical residues in soil and water samples.
References
- https://www.slideshare.net/shaisejacob/paper-chromatography-pptnew?next_slideshow=1
- https://www.slideshare.net/shaisejacob/paper-chromatography-ppt-new
- https://www.biochemden.com/paper-chromatography/
- https://microbenotes.com/paper-chromatography/
- https://pubs.acs.org/doi/abs/10.1021/ac60051a002
Other related topics you might be interested in:
Chromatography – Principle, Types, Steps, Uses, and Advantages
Adsorption Chromatography – Principle, Types, Procedure, Applications & Advantages
Affinity Chromatography – Principle, Components, Procedure, Applications & Advantages
Gas Chromatography (GC) – Principle, Parts, Procedure, Steps, Applications, Advantages & Limitations
High-Performance Liquid Chromatography (HPLC) – Principle, Instrumentation, Types & Applications
Ion Exchange Chromatography – Principle, Instrumentation, Procedure, Applications
Thin Layer Chromatography (TLC) – Principle, Steps, Applications, Advantages & Limitations