Introduction to UV Spectroscopy
- Spectroscopy is the study of the interaction between matter and electromagnetic radiation.
- In UV spectroscopy, light from the ultraviolet (UV) region (200–400 nm) is absorbed by molecules, causing electronic excitation (promotion of electrons from ground state to higher energy states).
- Molecules with π-electrons (like in double bonds) or non-bonding electrons (n-electrons) absorb UV light strongly.
- The technique produces a unique absorption spectrum that serves as a molecular fingerprint for qualitative and quantitative analysis.
In simple terms: UV Spectroscopy tells us about the structure, purity, and concentration of compounds by analyzing how they absorb UV light.

Principle of UV Spectroscopy
- When a molecule absorbs UV light, its electrons are excited from ground state (low energy) to excited state (high energy).
- The wavelength of absorbed light depends on how easily electrons can be excited.
- Molecules with conjugated double bonds absorb at longer wavelengths.
- The absorption spectrum helps identify compounds and functional groups.
Types of Electronic Transitions in UV Spectroscopy
- σ → σ* (highest energy transition)
- Involves excitation of sigma bond electrons.
- Requires strong UV light of shorter wavelength (<200 nm).
- n → σ*
- Involves excitation of non-bonding electrons (lone pairs) to antibonding σ-orbitals.
- Absorption occurs in 150–250 nm region.
- π → π*
- Involves excitation of pi electrons in double bonds to antibonding π-orbitals.
- Found in molecules with C=C, C=O, and aromatic systems.
- n → π*
- Involves excitation of lone pairs to π* antibonding orbitals.
- Seen in carbonyl groups (C=O) and compounds with heteroatoms.
- Requires less energy, absorbs at longer wavelengths.
Order of energy required: σ → σ* > n → σ* > π → π* > n → π*
Components of UV Spectroscopy
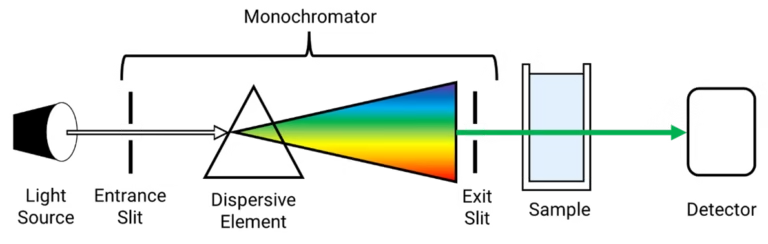
A UV spectrophotometer has the following main parts:
- Radiation Source
- Provides UV light (200–400 nm).
- Common sources:
- Deuterium lamp – for continuous UV radiation.
- Hydrogen lamp – alternative source.
- Monochromator
- Splits light into individual wavelengths.
- Uses prisms or diffraction gratings.
- Sample Holder (Cuvette)
- Usually made of quartz (since glass absorbs UV).
- Holds liquid samples in path of UV light.
- Detector
- Measures transmitted light after passing through sample.
- Common detectors:
- Photomultiplier tube
- Photodiode array detectors
- Recorder/Display Unit
- Converts detector signals into a spectrum.
- Produces a graph of absorbance vs wavelength.
Steps in UV Spectroscopy
- Sample Preparation
- Dissolve sample in a solvent transparent to UV light (e.g., ethanol, hexane).
- Place in a quartz cuvette.
- Radiation Exposure
- Pass UV light through the sample.
- Some light is absorbed, the rest is transmitted.
- Detection
- Detector measures transmitted light.
- Data Analysis
- The spectrophotometer generates a graph (spectrum) showing peaks at absorbed wavelengths.
- The position and intensity of peaks provide structural information.
Factors Affecting UV Absorption
- Conjugation: Longer conjugation → absorption shifts to longer wavelengths (bathochromic shift).
- Polarity of Solvent: Polar solvents stabilize excited states → shift absorption peaks.
- Substituents: Electron-donating groups shift absorption to longer wavelengths.
- pH: Ionization of molecules can change absorption.
Applications of UV Spectroscopy
1. Detection of Impurities
- Extra peaks in the spectrum indicate impurities.
- Comparison with pure standard helps identify contamination.
2. Structure Elucidation of Organic Compounds
- Identifies functional groups and conjugated systems.
- Helps determine unsaturation and presence of heteroatoms.
3. Qualitative and Quantitative Analysis
- Qualitative: Identification by comparing spectra with reference.
- Quantitative: Based on Beer-Lambert’s law (Absorbance = εcl).
4. Drug Analysis in Pharmaceuticals
- Used to assay raw materials and drug formulations.
- Quick method for quality control of medicines.
5. Kinetics of Reactions
- Absorbance changes with time → used to study reaction rates.
6. Molecular Weight Determination
- By preparing derivatives and analyzing absorbance, approximate molecular weight can be determined.
7. Chromatography Detector
- UV detectors are widely used in HPLC for compound detection.
Advantages of UV Spectroscopy
- Simple and rapid technique.
- Non-destructive method.
- Requires very small sample.
- High sensitivity.
- Applicable to both qualitative and quantitative analysis.
- Can detect impurities at low concentrations.
Limitations of UV Spectroscopy
- Only works for compounds with chromophores.
- Provides limited structural information (not as detailed as NMR or IR).
- Overlapping spectra may complicate interpretation.
- Sample must be soluble in UV-transparent solvent.
FAQs on UV Spectroscopy
Q1. What is UV Spectroscopy?
Ans: It is an analytical method that studies how molecules absorb ultraviolet light (200–400 nm) to identify and analyze compounds.
Q2. What is the principle of UV Spectroscopy?
Ans: Molecules absorb UV radiation, causing electronic excitation of π and n electrons to higher energy states.
Q3. What type of transitions are studied in UV Spectroscopy?
Ans: σ → σ*, n → σ*, π → π*, and n → π*.
Q4. Why is quartz used in UV cuvettes instead of glass?
Ans: Glass absorbs UV light, while quartz is transparent to it.
Q5. What are the applications of UV Spectroscopy?
Ans: Drug analysis, impurity detection, reaction kinetics, structure elucidation, and as detectors in chromatography.
Q6. What is Beer-Lambert’s law in UV Spectroscopy?
Ans: It relates absorbance (A) to concentration (c), path length (l), and molar absorptivity (ε): A = εcl.
Q7. Can UV Spectroscopy be used for quantitative analysis?
Ans: Yes. It is widely used for quantitative estimation of compounds using Beer-Lambert’s law.
Q8. What are chromophores and auxochromes in UV Spectroscopy?
Ans:
- Chromophores: Groups responsible for absorption (C=C, C=O, aromatic rings).
- Auxochromes: Groups that modify absorption intensity or wavelength (–OH, –NH2).
Q9. What is a bathochromic shift?
Ans: Also called red shift, it is the shift of absorption to longer wavelength due to conjugation or solvent effects.
Q10. What is a hypsochromic shift?
Ans: Also called blue shift, it is the shift of absorption to shorter wavelength, usually due to loss of conjugation.
Q11. Can UV spectroscopy detect impurities in drugs?
Ans: Yes. It is one of the most reliable methods for detecting impurities in pharmaceuticals.
Q12. What solvents are used in UV Spectroscopy?
Ans: Ethanol, hexane, cyclohexane, and water (if transparent in the UV region).
Q13. What are the advantages of UV Spectroscopy?
Ans: Rapid, simple, sensitive, non-destructive, and requires small sample volume.
Q14. What are the limitations of UV Spectroscopy?
Ans: Cannot analyze compounds without chromophores, less detailed structural information compared to IR or NMR.
Q15. Why is UV Spectroscopy important in pharmaceuticals?
Ans: It ensures drug purity, potency, and quality control during manufacturing.
Conclusion
- UV Spectroscopy is a fundamental analytical tool in chemistry, biology, and pharmaceuticals.
- It works on the principle of electronic transitions caused by UV light absorption.
- Its applications range from impurity detection and drug analysis to chromatography detection and reaction kinetics.
- Although limited in providing detailed structural data, its simplicity, sensitivity, and speed make it indispensable.
In short: UV Spectroscopy is a fast, reliable, and widely used technique for studying molecular properties through light absorption.
References
- http://www.indiastudychannel.com/resources/146681-Principle-working-and-applications-of-UV-spectroscopy.aspx
- https://microbenotes.com/uv-spectroscopy-principle-instrumentation-applications/
- https://www.slideshare.net/manishpharma/application-of-uv-spectroscopy
- https://en.wikipedia.org/wiki/Ultraviolet%E2%80%93visible_spectroscopy
Other related topics you might be interested in:
Mass Spectrometry – Principle, Steps, Instrumentation, Types & Applications
Spectrophotometer – Principle, Components, Applications, Advantages & Limitations
Types of Spectroscopy – Principles, Types, Steps, and Applications
UV-Vis Spectroscopy – Principle, Instrumentation, Applications, Advantages & Limitations
