What are the differences between Osmosis and Diffusion?
Following are the differences between Osmosis and Diffusion -:
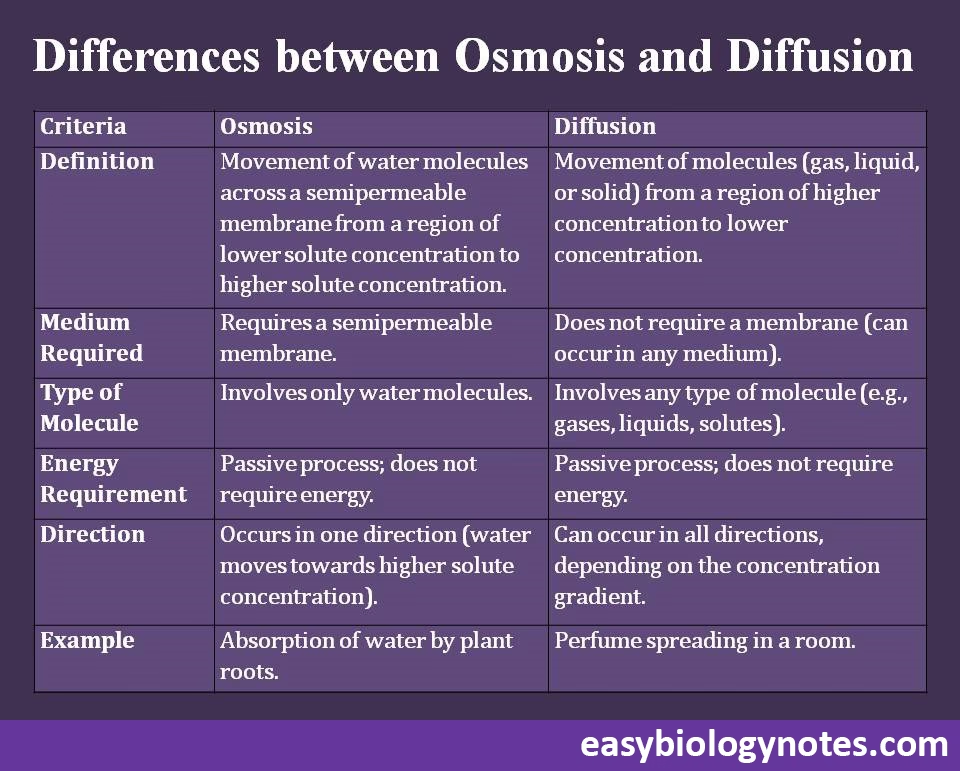
| Criteria | Osmosis | Diffusion |
| Definition | Movement of water molecules across a semipermeable membrane from a region of lower solute concentration to higher solute concentration. | Movement of molecules (gas, liquid, or solid) from a region of higher concentration to lower concentration. |
| Medium Required | Requires a semipermeable membrane. | Does not require a membrane (can occur in any medium). |
| Type of Molecule | Involves only water molecules. | Involves any type of molecule (e.g., gases, liquids, solutes). |
| Energy Requirement | Passive process; does not require energy. | Passive process; does not require energy. |
| Direction | Occurs in one direction (water moves towards higher solute concentration). | Can occur in all directions, depending on the concentration gradient. |
| Example | Absorption of water by plant roots. | Perfume spreading in a room. |
Elaborative notes on Differences Between Osmosis and Diffusion
Osmosis and diffusion are fundamental processes that govern the movement of molecules in biological and physical systems. Both are passive transport mechanisms, requiring no external energy input, but they differ in the type of molecules they transport, the mechanisms they utilize, and their specific roles in maintaining cellular and systemic homeostasis. These processes are essential for the survival of living organisms, influencing everything from cellular hydration to gas exchange and nutrient distribution.
Understanding the differences between osmosis and diffusion is not only important for biological sciences but also for applications in fields like medicine, environmental science, and industry.
1. Mechanism of Molecule Movement
- Diffusion is the movement of molecules or ions from an area of higher concentration to an area of lower concentration, driven by the principle of equilibrium. This process occurs across concentration gradients until uniform distribution is achieved. Diffusion can occur with various substances, including gases, liquids, and dissolved solutes. For instance, oxygen diffusing into cells and carbon dioxide diffusing out are critical for respiration.
- Osmosis, on the other hand, is the movement of water molecules specifically across a selectively permeable membrane. It occurs when there is a difference in solute concentration on either side of the membrane, causing water to move from a region of lower solute concentration (hypotonic) to a region of higher solute concentration (hypertonic). Osmosis is essential for regulating water balance in cells and maintaining turgor pressure in plant cells.
2. Type of Molecules Involved
- Diffusion involves a wide range of substances, including gases like oxygen and carbon dioxide, small solutes, and ions. It is not limited to water and can occur in various mediums, including air and liquid. For example, the exchange of oxygen and carbon dioxide in the lungs during respiration is an example of gaseous diffusion.
- Osmosis is strictly concerned with the movement of water molecules. This specificity makes osmosis a unique and vital process for maintaining cellular hydration and volume. In biological systems, osmosis ensures that cells do not shrink or swell excessively, which could compromise their function or integrity.
3. Role of Membranes
- Diffusion can occur with or without a membrane. In cases where a membrane is involved, it need not be selectively permeable; for instance, diffusion can happen across open surfaces, like the alveoli in lungs.
- Osmosis, however, requires a selectively permeable membrane that allows the passage of water molecules while restricting the movement of solutes. The semi-permeability of the membrane is critical, as it enables the cell to control its internal environment, maintaining optimal conditions for enzymatic and metabolic activity.
4. Biological Significance
- Diffusion plays a universal role in transporting substances across biological membranes. For example, in cells, the diffusion of nutrients, waste products, and respiratory gases ensures efficient cellular function. The movement of ions through ion channels, governed by diffusion, is critical for processes like nerve impulse transmission and muscle contraction.
- Osmosis is crucial for water balance and pressure regulation within cells and tissues. In plants, osmosis helps maintain turgor pressure, which supports the upright position of stems and leaves. In animals, osmoregulation is vital for maintaining the fluid balance in blood and tissues, directly influencing blood pressure and kidney function.
5. Rate and Regulation
- The rate of diffusion is influenced by factors such as the concentration gradient, temperature, molecular size, and the medium through which diffusion occurs. For example, diffusion occurs more rapidly in gases than in liquids due to the greater kinetic energy of gas molecules.
- The rate of osmosis is affected by the solute concentration gradient, the water potential, and the permeability of the membrane. Osmosis is more regulated in biological systems, often controlled by mechanisms like aquaporins—specialized membrane proteins that facilitate water transport.
6. Applications Beyond Biology
- Diffusion is widely utilized in various fields. In environmental science, diffusion helps explain the dispersion of pollutants in the air or water. In medicine, diffusion is fundamental to drug delivery, where medications are designed to diffuse efficiently to target cells.
- Osmosis has applications in water purification and desalination processes, where osmotic principles are used to separate water from solutes. Reverse osmosis systems are particularly important for producing potable water in areas with limited freshwater availability.
7. Cellular Implications and Adaptations
- In hypertonic environments, osmosis causes cells to lose water and shrink, a process called plasmolysis in plants. Conversely, in hypotonic environments, cells may swell and burst due to excessive water intake. Cells have evolved mechanisms to counteract these effects, such as contractile vacuoles in freshwater organisms, which expel excess water.
- Diffusion limitations in larger organisms have led to the development of specialized structures like lungs and circulatory systems. These systems optimize the diffusion of gases and nutrients, ensuring efficient exchange even in multicellular organisms.
8. Evolutionary Perspective
- Diffusion and osmosis highlight the elegance of natural processes that require no external energy yet are crucial for maintaining life. The evolution of selectively permeable membranes and transport mechanisms has allowed organisms to adapt to diverse environments. These processes underline the importance of passive transport in the early origins of life, when cells relied solely on environmental gradients for nutrient uptake and waste removal.
Conclusion
In conclusion, osmosis and diffusion are integral to the functioning of all living organisms, reflecting the interplay between simplicity and necessity in biological systems. Diffusion provides a fundamental means of distributing molecules across concentration gradients, while osmosis ensures the precise regulation of water within cells and tissues. Together, these processes maintain homeostasis, enabling organisms to survive and thrive in varying environmental conditions.
The study of osmosis and diffusion has far-reaching implications, from understanding basic cellular physiology to developing innovative technologies for healthcare and environmental sustainability. As we delve deeper into these mechanisms, we uncover not only their importance in biology but also their relevance to solving global challenges, such as water scarcity and efficient drug delivery. Ultimately, osmosis and diffusion exemplify the profound simplicity underlying complex biological systems, showcasing the intricate balance that sustains life.