Introduction to Gas Chromatography
- Gas Chromatography (GC) is a powerful analytical technique used to separate, identify, and quantify compounds that can exist in the gaseous phase.
- Unlike other types of chromatography where the mobile phase is a liquid, in GC the mobile phase is a gas (usually helium, nitrogen, or hydrogen).
- The sample to be analyzed must either be a gas or a liquid that can be vaporized.
- GC is commonly used for volatile and thermally stable compounds.
In simple words: Gas Chromatography separates a mixture into its components by making them travel through a column with the help of an inert carrier gas.

Principle of Gas Chromatography
The principle of GC is based on partitioning between:
- Mobile Phase (Carrier Gas) – moves the sample through the column.
- Stationary Phase (liquid or solid coated on the column surface) – interacts differently with each component.
- Compounds with higher affinity for stationary phase stay longer in the column and elute later (longer retention time).
- Compounds with greater affinity for carrier gas elute faster (shorter retention time).
- Separation depends on:
- Polarity of stationary phase
- Boiling point of the compound
- Intermolecular interactions
Each separated compound appears as a peak on the chromatogram, and its retention time (Rt) is used for identification.
Parts of Gas Chromatography Instrument
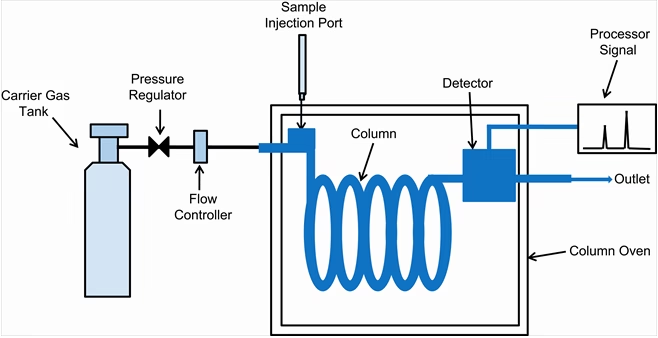
A GC instrument has several important parts:
1. Carrier Gas Supply
- Acts as the mobile phase.
- Common gases: Helium, Nitrogen, Hydrogen, Argon.
- Chosen for being inert and not reacting with the sample.
- Flow is controlled using pressure regulators and flow meters.
2. Sample Injection System
- Small amounts of sample (0.1–0.2 mL) are injected.
- Liquid samples → injected using a microsyringe into a heated injection port (to vaporize).
- Gas samples → injected using a gas-tight syringe.
- Injection must be fast to ensure all sample enters at once.
3. Column (Separation Column)
- The heart of gas chromatography.
- Long tubes (metal or glass) coiled in spiral or U-shape.
- Types of phases:
- Non-polar phases → separate based on boiling points.
- Intermediate polarity phases → separate both polar & non-polar compounds.
- Polar phases → separate polar compounds (e.g., alcohols, amines).
- Special phases → achieve separation via chemical reactions (e.g., AgNO₃ for unsaturated hydrocarbons).
4. Supports
- Material that holds the stationary phase.
- Should be inert, porous, and provide large surface area.
- Examples: diatomaceous earth, porous polymer beads.
5. Detector
- Detects and records compounds as they leave the column.
- Produces a signal converted into peaks on the chromatogram.
- Common detectors:
- Thermal Conductivity Detector (TCD)
- Flame Ionization Detector (FID)
- Electron Capture Detector (ECD)
- Mass Spectrometer (MS) (when coupled with GC).
6. Recorder / Data System
- Records detector signals.
- Produces a chromatogram (graph of time vs detector response).
- Peak area → proportional to compound concentration.
Steps / Procedure of Gas Chromatography
Step 1: Sample Injection & Vaporization
- Sample is injected into the hot injection port.
- Quickly vaporizes and mixes with carrier gas.
Step 2: Separation in the Column
- Components partition between mobile phase and stationary phase.
- Stronger binding → longer retention time (elutes later).
- Weaker binding → shorter retention time (elutes earlier).
Step 3: Detection & Recording
- Separated compounds reach the detector at different times.
- Detector produces electrical signals → displayed as peaks.
- Chromatogram is analyzed for:
- Retention time (identity of compound).
- Peak area/height (concentration of compound).
Applications of Gas Chromatography
GC is one of the most widely used analytical tools in science and industry.
- Environmental Analysis
- Detect air pollutants and toxic gases.
- Measure pesticides in soil and water.
- Analyze oil spills.
- Forensic Science
- Drug analysis in urine or blood.
- Arson investigation (accelerants in fire debris).
- Paint, ink, and fiber analysis.
- Pharmaceutical & Medical Applications
- Detect impurities in drugs.
- Measure blood alcohol content.
- Analyze biological fluids (e.g., hormones, fatty acids).
- Food & Flavor Industry
- Quality testing of beverages.
- Analysis of essential oils and perfumes.
- Detect additives and preservatives.
- Chemical Industry
- Monitor chemical production.
- Identify unknown compounds.
- Purity testing of raw materials.
Advantages of Gas Chromatography
- Very accurate, sensitive, and precise.
- Separations are fast (minutes).
- Can detect very small amounts (ppb level).
- Works at high temperatures (up to 500°C).
- Wide range of applications (environmental, medical, industrial).
- Can be coupled with Mass Spectrometry (GC-MS) for more detailed analysis.
Limitations of Gas Chromatography
- Only suitable for compounds that are:
- Volatile
- Thermally stable
- Low molecular weight (<1000 Da)
- Non-volatile or salt-containing samples cannot be analyzed.
- Requires comparison with reference standards for identification.
- Equipment is expensive and requires trained operators.
Precautions for Using Gas Chromatography
- Use high purity carrier gases to avoid contamination.
- Keep the injection port and column at correct temperatures.
- Avoid injecting too much sample (can overload the column).
- Regular maintenance of detector and column is necessary.
- Handle gas cylinders with proper safety precautions.
FAQs on Gas Chromatography
Q1. Why is helium commonly used as carrier gas?
Ans: Because it is inert, has low molecular weight, and provides good resolution.
Q2. What is retention time in GC?
Ans: The time taken by a compound to pass through the column and reach the detector.
Q3. Can GC analyze proteins or DNA?
Ans: No, GC is only suitable for small, volatile, and thermally stable molecules.
Q4. What is GC-MS?
Ans: Combination of Gas Chromatography with Mass Spectrometry → provides both separation and molecular identification.
Q5. What are the main detectors used in GC?
Ans: TCD, FID, ECD, and MS are the most common detectors.
Conclusion
Gas Chromatography (GC) is a versatile analytical technique that plays a crucial role in environmental science, food safety, forensics, pharmaceuticals, and research.
By understanding its principle, components, procedure, applications, advantages, and limitations, students and researchers can better appreciate its importance in modern laboratories.
In simple words, Gas Chromatography helps us separate, identify, and measure even the smallest traces of compounds with high accuracy.
References
- https://nptel.ac.in/courses/103108100/module7/module7.pdf
- https://en.wikipedia.org/wiki/Gas_chromatography#Carrier_gas_selection_and_flow_rates
- http://web.uniplovdiv.bg/plamenpenchev/mag/books/anchem/Handbook%20of%20Analytical%20Techniques,%202%20Volume%20Set.pdf
Other related topics you might be interested in:
Chromatography – Principle, Types, Steps, Uses, and Advantages
Adsorption Chromatography – Principle, Types, Procedure, Applications & Advantages
Affinity Chromatography – Principle, Components, Procedure, Applications & Advantages
High-Performance Liquid Chromatography (HPLC) – Principle, Instrumentation, Types & Applications
Ion Exchange Chromatography – Principle, Instrumentation, Procedure, Applications
Paper Chromatography – Definition, Principle, Types, Steps, Applications, Advantages & Limitations
Thin Layer Chromatography (TLC) – Principle, Steps, Applications, Advantages & Limitations