Introduction
Chromatography is one of the most essential analytical techniques in biology and chemistry. It helps to separate complex mixtures into their individual components. Among its many types, Ion Exchange Chromatography (IEC or IC) is widely used for separating ions and charged molecules such as amino acids, proteins, nucleic acids, and inorganic ions.
In this method, molecules are separated based on their charge interactions with a stationary phase (ion exchanger). Because of its efficiency and versatility, ion exchange chromatography is used in biochemistry, water purification, medicine, environmental monitoring, and industrial processes.

Principle of Ion Exchange Chromatography
- The method works on the reversible exchange of ions between the charged groups of the stationary phase (ion exchanger) and the ions present in the sample.
- There are two types of ion exchangers:
- Cation exchangers – Have negatively charged groups that attract positively charged cations.
- Anion exchangers – Have positively charged groups that attract negatively charged anions.
- The separation depends on the strength of ionic interaction between molecules and the stationary phase.
- Molecules with higher affinity to the exchanger bind strongly, while weaker ones elute earlier.

Example:
- DEAE-cellulose is a common anion exchanger.
- CM-cellulose is a common cation exchanger.
Working Principle – Step by Step
- Column Preparation
- A glass, stainless steel, or plastic column is packed with an ion exchanger.
- Sample Loading
- The sample containing charged molecules is applied to the column.
- Binding of Ions
- Ions in the sample bind to the stationary phase depending on charge.
- Washing
- A buffer is passed through the column to remove unbound molecules.
- Common buffers: phosphate buffer, acetate buffer, tris buffer.
- Elution of Bound Molecules
- Bound ions are released by changing buffer pH or ionic strength (salt gradient).
- Strongly bound molecules require stronger conditions to elute.
- Detection & Analysis
- Eluted molecules are detected, usually by spectroscopy or conductivity detectors.
Instrumentation of Ion Exchange Chromatography
A standard Ion Chromatography system has the following parts:
1. Pump
- Delivers the mobile phase (buffer) at constant flow.
- Must handle high pressures (up to 4000 psi).
2. Injector
- Introduces the sample into the column.
- Can inject 0.1 – 100 ml of liquid sample.
3. Column
- Heart of the system where separation occurs.
- Made of stainless steel, glass, or inert plastics (PEEK).
- A guard column is often placed before the main column to protect it from impurities.
4. Suppressor
- Reduces background conductivity of buffers.
- Converts ionic eluent into water for higher sensitivity.
5. Detector
- The most common detector is the conductivity detector, which measures ion concentration.
- UV detectors may also be used for biomolecules.
6. Data System / Recorder
- Modern systems use computer-based software to record chromatograms and quantify results.
Types of Ion Exchangers
- Cation Exchangers
- Contain negatively charged groups (e.g., carboxymethyl cellulose).
- Attract and bind positively charged ions.
- Anion Exchangers
- Contain positively charged groups (e.g., DEAE cellulose).
- Attract and bind negatively charged ions.
Procedure of Ion Exchange Chromatography
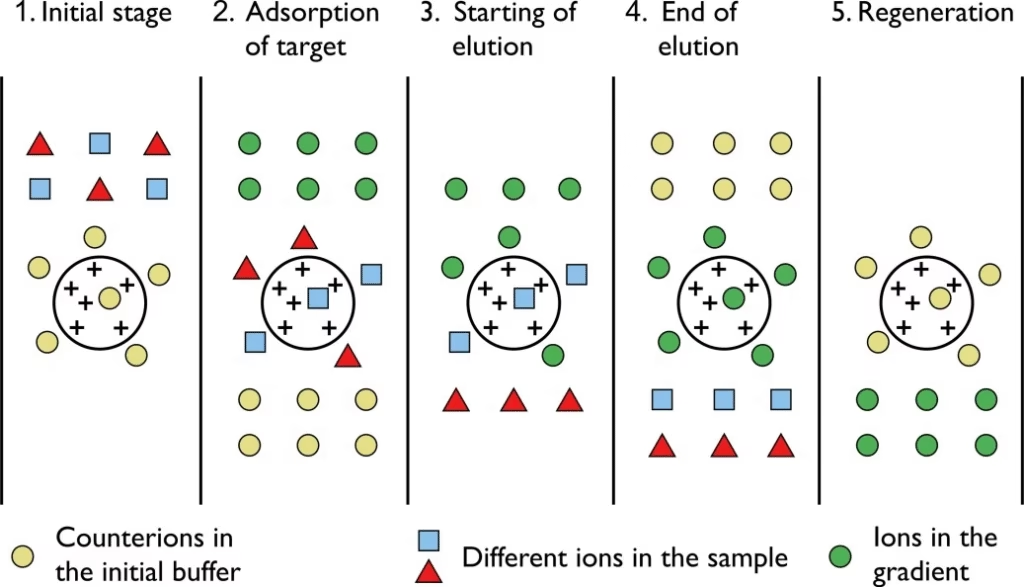
- Pack the column with ion exchanger material.
- Equilibrate the column with an appropriate buffer.
- Load the sample solution.
- Wash unbound molecules using the same buffer.
- Elute bound molecules by changing pH or adding salt gradient.
- Collect fractions and analyze them (e.g., spectrophotometry).
Applications of Ion Exchange Chromatography
1. In Biology & Biochemistry
- Separation of amino acids, proteins, nucleotides, and nucleic acids.
- Protein purification for research and medicine.
- DNA and RNA analysis.
2. In Medicine
- Clinical diagnosis of amino acid disorders.
- Production and purification of therapeutic proteins.
- Quality control in pharmaceuticals.
3. In Environmental Science
- Water purification and softening (removing Ca²⁺, Mg²⁺, Cl⁻, SO₄²⁻).
- Detecting and removing toxic heavy metals from wastewater.
4. In Chemistry
- Separation of inorganic and organic ions.
- Analysis of hydrolyzed nucleic acids.
- Tracing rare earth elements and metals.
5. In Space & Geology
- Analyzing lunar rocks and rare trace elements.
- Collection of trace metals from seawater.
Advantages of Ion Exchange Chromatography
- Highly efficient method for separating charged molecules.
- Can handle small as well as large biomolecules.
- Suitable for both analytical and preparative purposes.
- Inorganic as well as organic ions can be separated.
- High sensitivity and reproducibility.
Limitations of Ion Exchange Chromatography
- Only charged molecules can be separated.
- Requires buffer systems and careful pH control.
- Column efficiency may reduce due to fouling or clogging.
- Strongly bound ions may need harsh conditions to elute.
Frequently Asked Questions (FAQ)
Q1. What is Ion Exchange Chromatography in simple words?
It is a method to separate charged molecules (like proteins, amino acids, or ions) using columns packed with charged materials called ion exchangers.
Q2. What is the difference between cation and anion exchange chromatography?
- Cation exchange uses negatively charged exchangers to separate positively charged molecules.
- Anion exchange uses positively charged exchangers to separate negatively charged molecules.
Q3. What buffers are used in Ion Exchange Chromatography?
Commonly used buffers are phosphate, tris, acetate, citrate, and pyridine buffers.
Q4. What is a suppressor in Ion Chromatography?
It reduces the background conductivity of the eluent and increases the sensitivity of detection.
Q5. What is Ion Exchange Chromatography used for?
It is used for protein purification, amino acid analysis, nucleic acid studies, water purification, and environmental monitoring.
References
- Wilson, K., Walker, J. (2018). Principles and Techniques of Biochemistry and Molecular Biology (8 eds.). Cambridge University Press: New York.
- https://www.biochemden.com/ion-exchange-chromatography/
- https://www.britannica.com/science/ion-exchange-reaction/Applications-of-ion-exchange
- http://cdn.intechopen.com/pdfs/43603/InTech-Ion_exchange_chromatography_an_overview.pdf
Other related topics you might be interested in:
Chromatography – Principle, Types, Steps, Uses, and Advantages
Adsorption Chromatography – Principle, Types, Procedure, Applications & Advantages
Affinity Chromatography – Principle, Components, Procedure, Applications & Advantages
Gas Chromatography (GC) – Principle, Parts, Procedure, Steps, Applications, Advantages & Limitations
High-Performance Liquid Chromatography (HPLC) – Principle, Instrumentation, Types & Applications
Paper Chromatography – Definition, Principle, Types, Steps, Applications, Advantages & Limitations
Thin Layer Chromatography (TLC) – Principle, Steps, Applications, Advantages & Limitations
