Introduction to pH and pH Meter
- pH is the measure of acidity or alkalinity of a solution on a scale of 0 to 14.
- pH 7 = neutral, values below 7 = acidic, and values above 7 = alkaline/basic.
- pH is mathematically defined as:
pH = −log10[H+]
where [H+] = concentration of hydrogen ions (moles/L).
- In biology, chemistry, agriculture, and industry, pH measurement is essential for monitoring solutions, media, soil, food, and even biological fluids like blood and urine.
- A pH meter is a modern electronic device that measures hydrogen ion concentration in a solution and displays it directly as a pH value.
* Simply put: A pH meter tells us whether a solution is acidic, neutral, or basic with high accuracy.
Principle of pH Meter
The pH meter works on the principle of electrical potential difference created by hydrogen ions.
- The pH probe has two electrodes:
- Glass (sensor) electrode → sensitive to hydrogen ions.
- Reference electrode → provides a stable potential.
- When the electrode is dipped in a solution:
- Hydrogen ions interact with the glass membrane.
- This generates an electric potential compared to the reference electrode.
- The pH meter measures this voltage and converts it into a pH value.
* Key point: Higher acidity = higher voltage = lower pH reading. Higher alkalinity = lower voltage = higher pH reading.
Parts of a pH Meter
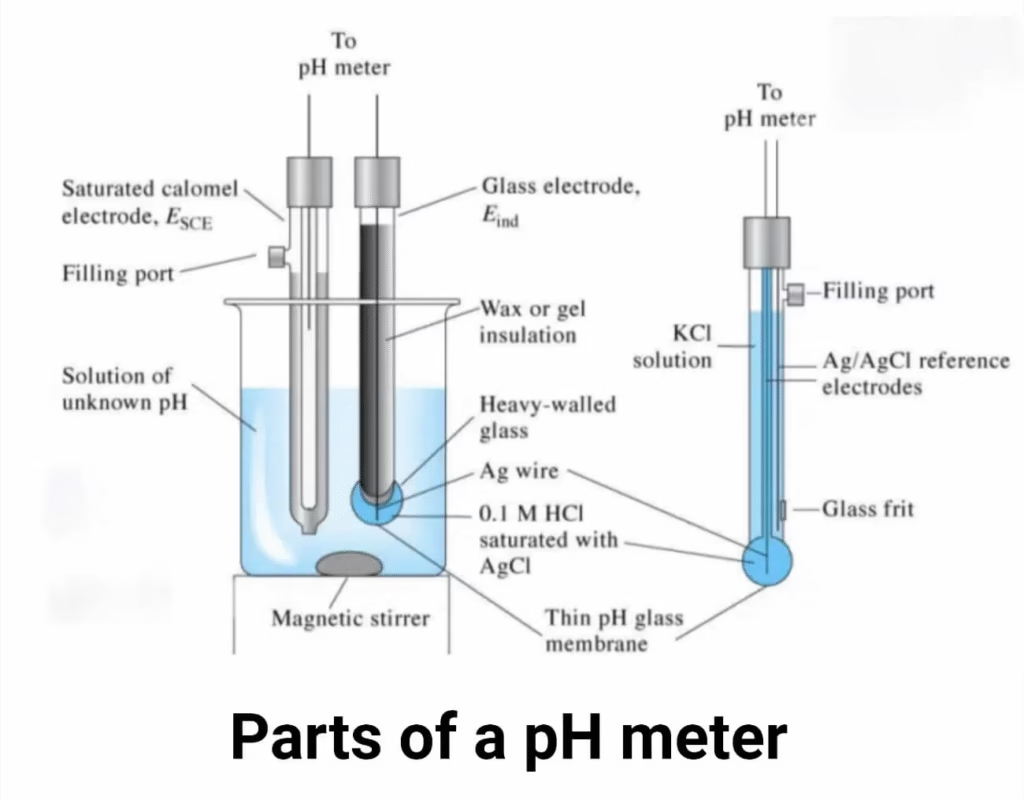
A typical pH meter has the following components:
1. High Input Impedance Meter
- Contains a microprocessor.
- Reads electrode voltage and converts it into a pH value.
- Also compensates for temperature effects.
2. Combined Electrode (pH Probe)
- Contains two electrodes:
- Reference electrode → stable voltage, usually filled with KCl solution.
- Glass electrode → sensitive to hydrogen ions, produces millivolt signals.
3. Amplifier
- Boosts weak electrode signals for accurate readings.
- Works like a thermometer amplifier for precision.
4. Thermometer Probe (ATC – Automatic Temperature Compensation)
- Some pH meters have temperature sensors.
- Since pH depends on temperature, this probe ensures more accurate results.
The probe is the most sensitive and expensive part of the pH meter. Handle it carefully.
Working / Operating Procedure of pH Meter
Step 1: Preparation
- Allow all samples to reach the same temperature.
- Calibrate the pH meter with standard buffer solutions (commonly pH 4, 7, and 10).
Step 2: Rinsing
- Rinse electrode with distilled/deionized water before use.
- Never use the same beaker for rinsing and measurement (to prevent contamination).
Step 3: Measurement
- Insert electrode into sample so that bulb and junction are fully immersed.
- Stir gently for uniform reading.
- Wait 1–2 minutes until the meter stabilizes.
- Record pH and temperature.
Step 4: Cleaning and Storage
- Rinse electrode again with distilled water.
- Store in electrode storage solution (not dry).
Types of pH Meters
pH meters can be classified in different ways:
A. Based on Portability
- Pen Testers
- Small, portable, inexpensive.
- Used in hydroponics, aquaculture, pools, and fieldwork.
- Handheld pH Meters
- More durable, separate electrode and display.
- Used in agriculture, water treatment, and environmental monitoring.
- Benchtop pH Meters
- Larger, highly accurate, used in laboratories.
- Common in research labs, food processing, and quality control.
B. Based on Usage
- Laboratory pH Meters → highly accurate, multipurpose.
- Industrial/Online pH Meters → monitor processes continuously in industries.
C. Based on Technology Level
- Economic pH Meters → simple, affordable.
- Intelligent pH Meters → advanced with digital output and ATC.
- Precision pH Meters → highly sensitive, available in analog and digital versions.
D. Based on Reading System
- Analog pH Meter → needle pointer display.
- Digital pH Meter → numerical display, easier to read and more accurate.
Applications of pH Meter
pH meters are widely used in biology, agriculture, medicine, and industry:
- Agriculture → Soil pH testing for crop suitability.
- Food Industry → Dairy, beverages, and packaged food quality testing.
- Water Treatment → Monitoring pH in drinking water, RO systems, and sewage treatment.
- Pharmaceuticals & Biotech → pH of drug solutions, media preparation.
- Chemical Industries → Neutralization of wastewater.
- Medical Field → pH of blood, urine, gastric juice, etc.
- Detergent Industry → pH control in manufacturing.
Advantages of pH Meter
- Highly accurate and precise compared to pH paper.
- Suitable for automatic continuous monitoring.
- Provides rapid and reproducible measurements.
- Works in colored, turbid, and colloidal solutions.
- Portable models are convenient for fieldwork.
- Does not alter the solution being tested.
Limitations of pH Meter
- Requires regular calibration with buffer solutions.
- Electrodes are fragile and expensive.
- Readings are affected by temperature variations.
- Deposits on electrode membranes can cause errors.
- Needs proper storage solution (cannot be kept dry).
Precautions While Using pH Meter
- Do not use the electrode as a stirring rod.
- Calibrate the pH meter daily with standard buffers.
- Avoid exposing the meter to direct sunlight/heat.
- Always rinse with distilled water before and after use.
- Use freshly prepared solutions for accurate readings.
Examples of pH Meters
- Laboratory pH Meter 1120X (Mettler Toledo) – high accuracy, chemical resistant.
- LAQUAtwin pH-11 – pocket-sized, requires only a drop of sample.
- PH DAY2 Gastroesophageal pH Meter – medical use for pH of gastric fluid.
- Portable pH Meter 913 (Metrohm) – waterproof, used in outdoor environments.

FAQs on pH Meter
Q1. Why is pH measurement important?
Ans: Because pH affects chemical reactions, biological processes, and product stability.
Q2. Which is better: pH paper or pH meter?
Ans: pH meters are more accurate and precise than pH papers.
Q3. Why is calibration important?
Ans: To ensure accuracy since electrodes can drift over time.
Q4. Can a pH meter measure temperature?
Ans: Yes, modern pH meters have ATC probes for temperature compensation.
Q5. What damages pH electrodes?
Ans: High temperatures, strong chemicals, and drying out of the glass bulb.
Conclusion
The pH meter is an essential scientific instrument for measuring the acidity or alkalinity of solutions with high precision. From soil testing in agriculture to monitoring blood pH in medicine, food safety, water treatment, and industrial processes, pH meters are widely used across multiple fields.
By understanding its principle, working, types, applications, and precautions, students and professionals can use pH meters effectively and maintain accuracy in experiments and industries.
References
- https://www.pharmaguideline.com/2015/08/principle-and-working-of-pH-probes.html
- https://microbiologie-clinique.com/laboratory-ph-meters.html
- https://assets.thermofisher.com/TFS-Assets/CMD/Product-Bulletins/TN-ph-calibration-procedure-for-optimal-measurement-precision-T-PHCAL-EN.pdf
- https://www.instrumentchoice.com.au/news/what-types-of-ph-meters-are-there
- https://www.medicalexpo.com/prod/horiba-scientific/product-104260-863724.html
Other related topics you might be interested in:
Bunsen Burner – Principle, Parts, Types, Flames, Applications, Advantages & Precautions
Centrifuge – Principle, Parts, Types, Operation, Applications and Advantages
Colony Counter – Definition, Principle, Types, Parts, Working, Applications, Advantages & Examples
Homogenizer – Principle, Parts, Types, Working, Procedure, Applications, Advantages & Limitations
Instruments Used in Microbiology Laboratory – Principles, Uses, and Applications
Laboratory Incubator – Principle, Types, Components, Working, Applications, Advantages & Limitations
Micropipette – Definition, Types, Parts, Working, Applications, Errors, Calibration & Limitations
Pipettes – Principle, Types, Uses, Parts, Operation, Advantages & Precautions
Ultracentrifuge – Principle, Types, Parts, Working Procedure, Applications, Advantages & Precautions
Vortex Mixer – Definition, Principle, Parts, Types, Working, Applications, Advantages & Precautions
