What is Chromatography?
- Chromatography is a widely used biophysical technique for separating, identifying, and purifying the components of a mixture.
- It works on the principle that different compounds distribute themselves differently between a stationary phase (does not move) and a mobile phase (moves in a definite direction).
- Depending on the nature of the stationary and mobile phases, chromatography can be of various types: paper chromatography, thin layer chromatography (TLC), gas chromatography, HPLC, etc.
In simple words: Chromatography helps in separating a mixture into its individual components for qualitative (type) and quantitative (amount) analysis.
What is Thin Layer Chromatography (TLC)?
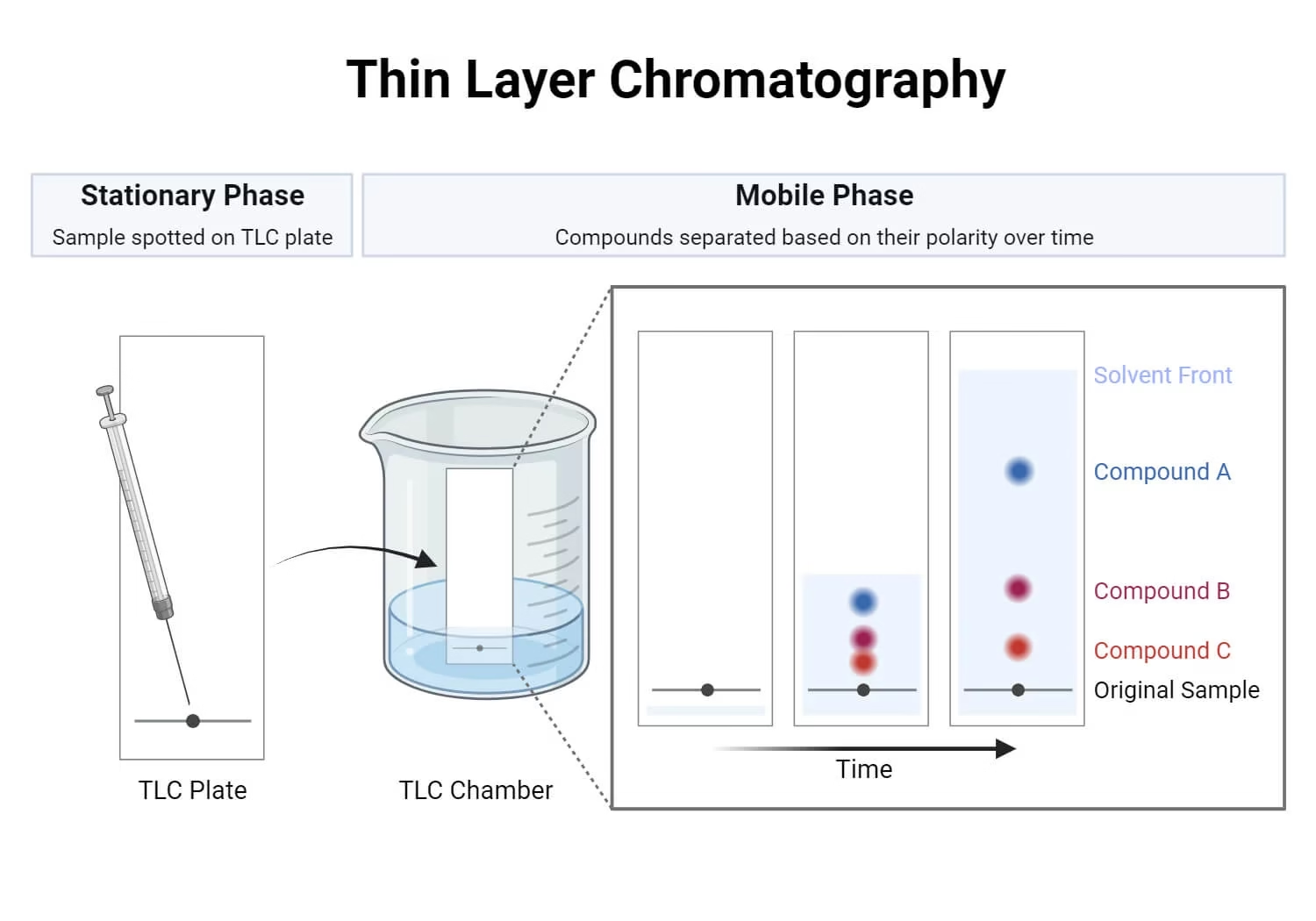
- Thin Layer Chromatography (TLC) is a simple, quick, and effective technique used to separate and identify the components of a mixture.
- It is performed on a plate coated with a thin layer of adsorbent material (e.g., silica gel, alumina, or cellulose) which acts as the stationary phase.
- A liquid solvent or mixture of solvents acts as the mobile phase.
- Different components of the sample move up the plate at different speeds due to varying affinities towards the stationary and mobile phases.
TLC is widely used because it is fast, inexpensive, and requires only small sample volumes.
Principle of Thin Layer Chromatography
- TLC is based on adsorption chromatography (sometimes partition chromatography depending on conditions).
- A thin layer of adsorbent (stationary phase) is coated on a plate.
- The sample mixture is spotted near the base of the plate.
- When placed in a chamber containing a solvent (mobile phase), the solvent moves up by capillary action.
- Components that interact strongly with the stationary phase move slowly, while those with weaker interactions move faster.
- This difference in mobility leads to separation of components.
After development, the separated spots are made visible using UV light, iodine vapors, or chemical reagents.
Components of a Thin Layer Chromatography System
A standard TLC setup includes:
- TLC Plate
- Usually made of glass, plastic, or aluminum foil coated with silica gel, alumina, or cellulose.
- Modern TLC plates are pre-coated and ready to use.
- TLC Chamber
- A closed container where development occurs.
- Maintains a saturated atmosphere to ensure even solvent movement.
- Prevents solvent evaporation and dust contamination.
- Mobile Phase (Solvent System)
- A single solvent or a mixture of solvents.
- Must be pure, chemically inert, and compatible with both sample and stationary phase.
- Filter Paper Lining
- Placed inside the chamber and moistened with mobile phase.
- Helps maintain uniform humidity and prevents edge effect.
- Sample Application Tools
- Samples are applied using micropipettes or capillary tubes at marked points near the plate’s bottom edge.
Steps of Thin Layer Chromatography
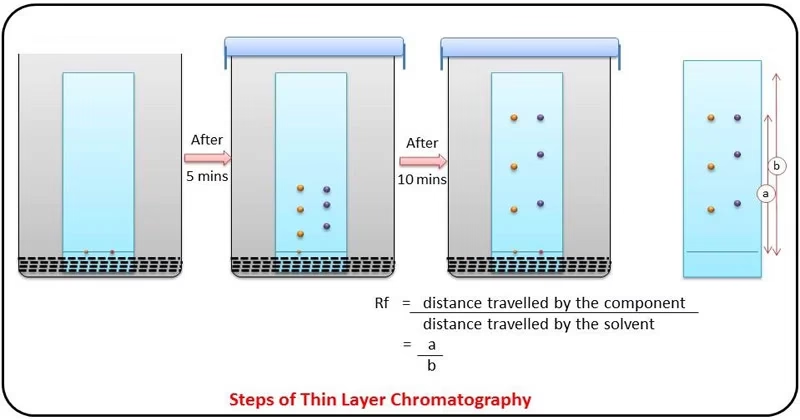
- Preparation of TLC Plate
- Use pre-coated TLC plates or coat manually with adsorbent material.
- Dry and activate plate by heating (to remove moisture).
- Sample Spotting
- Draw a pencil line near the bottom of the plate.
- Apply small drops of the sample solution with a capillary tube.
- Allow spots to dry before placing in chamber.
- Chamber Preparation
- Add mobile phase solvent into chamber (a few cm depth).
- Line chamber walls with moistened filter paper to maintain saturation.
- Development of TLC Plate
- Place the plate in chamber so that spots are above solvent level.
- Close lid to prevent evaporation.
- Allow solvent to rise until near the top of plate.
- Drying the Plate
- Remove plate and mark the solvent front.
- Allow plate to dry completely.
- Visualization of Spots
- Expose plate to UV light (254 nm or 365 nm).
- Use chemical stains:
- Iodine vapors – carbohydrates turn black.
- Ninhydrin reagent – detects amino acids.
- KMnO4 stain – detects organic molecules.
Retention Factor (Rf Value)
- The position of each spot is expressed as its Retention factor (Rf):
Rf = Distance traveled by compound/ Distance traveled by solvent front
- Rf values are unique for each compound under identical conditions.
- Factors affecting Rf values:
- Type of solvent system.
- Nature of adsorbent.
- Thickness of stationary phase.
- Amount of sample applied.
- Temperature.
Since Rf can vary, results are usually compared with a standard compound.
Applications of Thin Layer Chromatography
- In Chemistry and Biochemistry
- Monitoring progress of chemical reactions.
- Checking purity of compounds.
- Identifying unknown compounds by comparing Rf values.
- In Forensic Science
- Analyzing dyes in fibers and inks.
- Detecting drugs, toxins, and poisons.
- In Food and Agriculture
- Detection of pesticides and insecticides in food and water.
- Quality control of food colors, flavors, and preservatives.
- In Medicine & Pharmaceuticals
- Analyzing radiopharmaceuticals for purity.
- Identifying medicinal plants and their active compounds.
- Quality control of herbal drugs.
- In Environmental Science
- Detecting pollutants in water samples.
- Studying composition of soil extracts.
Advantages of Thin Layer Chromatography
- Simple, quick, and inexpensive technique.
- Requires very small sample volumes.
- Can separate a wide variety of compounds.
- High sensitivity – can detect small amounts.
- Works with colored and colorless compounds (with detection methods).
- Allows for simultaneous analysis of multiple samples.
- Useful for both qualitative and semi-quantitative analysis.
Limitations of Thin Layer Chromatography
- Cannot distinguish between enantiomers and some isomers.
- Requires known Rf values for compound identification.
- TLC plates are relatively short → limited resolution compared to HPLC.
- Sensitive to environmental factors (humidity, temperature).
- Results may not be highly reproducible.
Precautions in TLC
- Always use a pencil (not pen) to mark baseline.
- Apply small, concentrated spots to avoid smearing.
- Avoid dipping spots directly into solvent.
- Use freshly prepared and pure solvents.
- Keep chamber closed during development.
- Handle plates carefully to avoid scratching the adsorbent layer.
FAQs on Thin Layer Chromatography
Q1. Why is silica gel commonly used in TLC?
Ans: Because it is inexpensive, chemically inert, and has high adsorption capacity.
Q2. What is the difference between TLC and paper chromatography?
Ans: TLC uses a solid adsorbent coated plate, while paper chromatography uses paper (cellulose fibers) as stationary phase.
Q3. Can TLC be used for quantitative analysis?
Ans: Mostly TLC is qualitative, but densitometry methods allow semi-quantitative analysis.
Q4. Why is UV light used in TLC?
Ans: Many compounds fluoresce or absorb UV, making otherwise invisible spots detectable.
Q5. What is the typical Rf value range?
Ans: Between 0.0 and 1.0 (fraction of solvent front distance).
Conclusion
Thin Layer Chromatography (TLC) is a powerful, low-cost, and versatile separation technique. It allows quick analysis of mixtures, monitoring of reactions, detection of impurities, and identification of unknown compounds.
TLC is widely used in biological sciences, medicine, food quality testing, forensic science, pharmaceuticals, and environmental monitoring.
In short: TLC is one of the simplest yet highly effective chromatographic methods used in labs worldwide.
References
- https://owlcation.com/stem/tlc-thin-layer-chromatography-Principle-Procedure
- https://www.utsc.utoronto.ca/webapps/chemistryonline/production/tlc.php
- https://en.wikipedia.org/wiki/Thin-layer_chromatography
- https://chem.libretexts.org/Demos%2C_Techniques%2C_and_Experiments/General_Lab_Techniques/Thin_Layer_Chromatography
- https://www.slideshare.net/LavakusaBanavatu/thin-layer-chromatography-43293607
Other related topics you might be interested in:
Chromatography – Principle, Types, Steps, Uses, and Advantages
Adsorption Chromatography – Principle, Types, Procedure, Applications & Advantages
Affinity Chromatography – Principle, Components, Procedure, Applications & Advantages
Gas Chromatography (GC) – Principle, Parts, Procedure, Steps, Applications, Advantages & Limitations
High-Performance Liquid Chromatography (HPLC) – Principle, Instrumentation, Types & Applications
Ion Exchange Chromatography – Principle, Instrumentation, Procedure, Applications
Paper Chromatography – Definition, Principle, Types, Steps, Applications, Advantages & Limitations