Introduction to Chromatography
Chromatography is a widely used laboratory method for separating the components of a mixture based on how they interact with two phases — a stationary phase (which does not move) and a mobile phase (which moves).
- Each compound interacts differently with these phases, causing them to travel at different speeds and separate from one another.
- This separation makes it possible to identify, analyze, and purify different substances.
- Chromatography can be applied to a wide range of samples, from small molecules to large biomolecules.
The technique was first developed in 1906 by Russian botanist Mikhail Tswett, who used it to separate plant pigments. His experiment with a column packed with adsorbent material and a solvent led to colored bands separating within the column — the first example of chromatography in action.
Over time, chromatography has evolved into many different techniques, allowing scientists to analyze everything from air pollutants to DNA fragments.
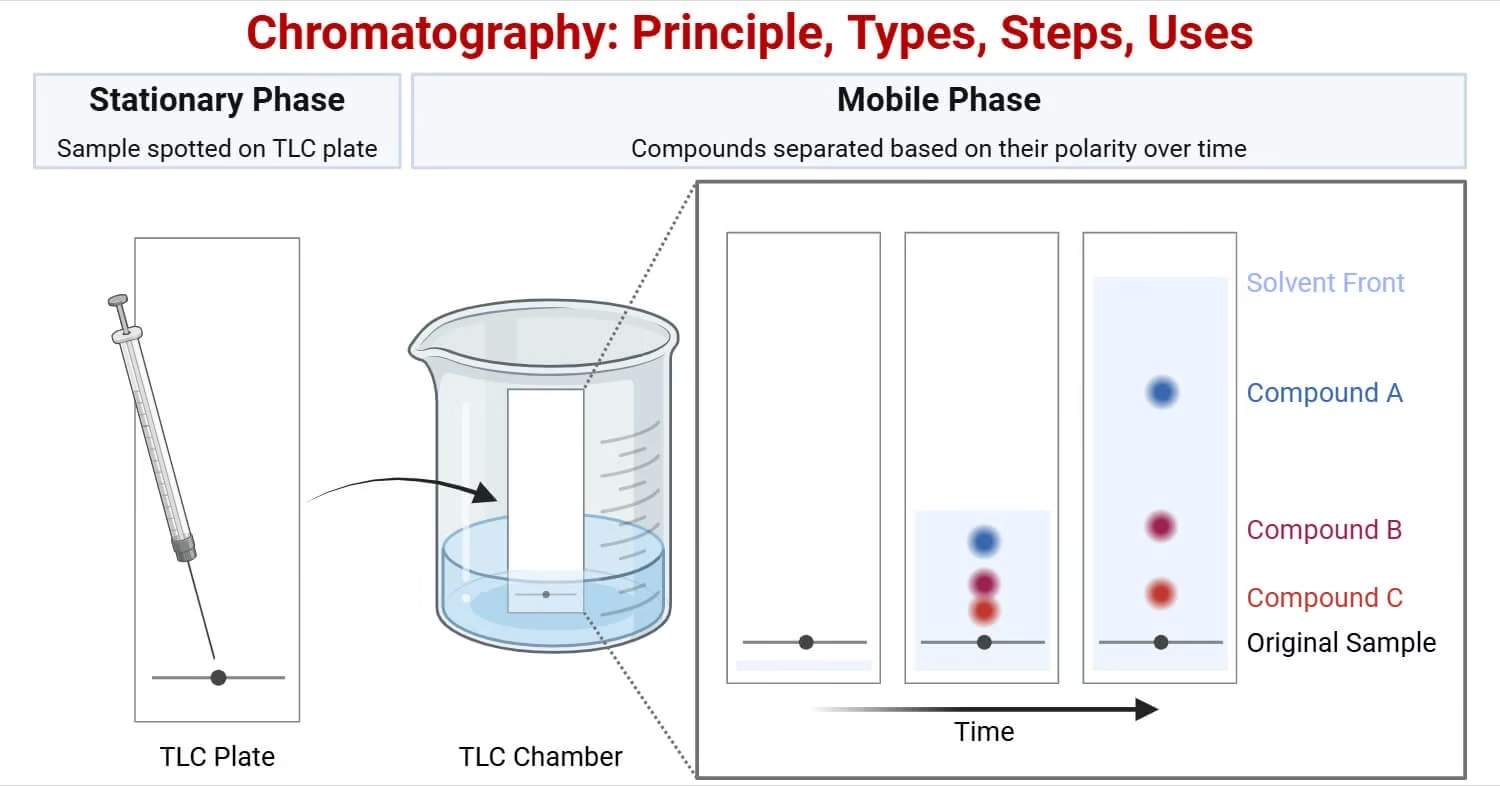
Principle of Chromatography
The principle of chromatography is based on the differential affinity of compounds toward the stationary and mobile phases.
- Stationary phase: Fixed in place (solid or liquid coating)
- Mobile phase: Moves and carries the sample through the stationary phase (liquid or gas)
When the mobile phase moves through the stationary phase:
- Compounds that interact strongly with the stationary phase move slower.
- Compounds that interact weakly move faster.
This difference in movement results in separation.
The separated substances are recorded as peaks on a chromatogram, and the retention time (time taken for a substance to pass through the system) helps identify them.
Main Components of a Chromatography System
- Sample (Analyte)
- The mixture to be separated.
- Each component behaves differently, depending on its chemical and physical properties.
- Stationary Phase
- Fixed material that slows or holds certain molecules.
- Examples: filter paper, silica gel, alumina, porous beads.
- Mobile Phase
- Moving fluid (liquid or gas) that carries the analyte.
- Examples: water, organic solvents, gases like helium, nitrogen, or hydrogen.
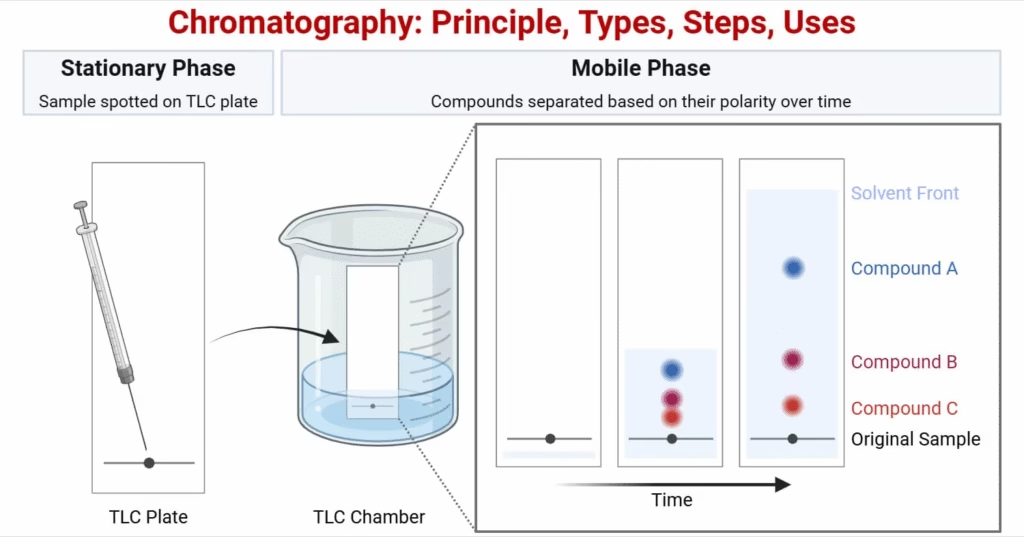
- Chromatography Column
- Container that holds the stationary phase.
- Used in column-based methods like HPLC and Gas Chromatography.
5. Detector
- Identifies and measures separated components.
- Common detectors: UV spectrophotometer, mass spectrometer, flame ionization detector.
Major Types of Chromatography
Chromatography can be classified based on geometry, separation mechanism, or phase type.
A. Based on Geometry
1. Planar Chromatography
- Stationary phase is spread on a flat surface.
- Mobile phase moves by capillary action.
- Examples: Paper Chromatography, Thin Layer Chromatography (TLC).
- Stationary phase: filter paper.
- Commonly used for plant pigments, dyes, and amino acids.
- The sample is spotted near the bottom of the paper, and the solvent moves upward, separating components.
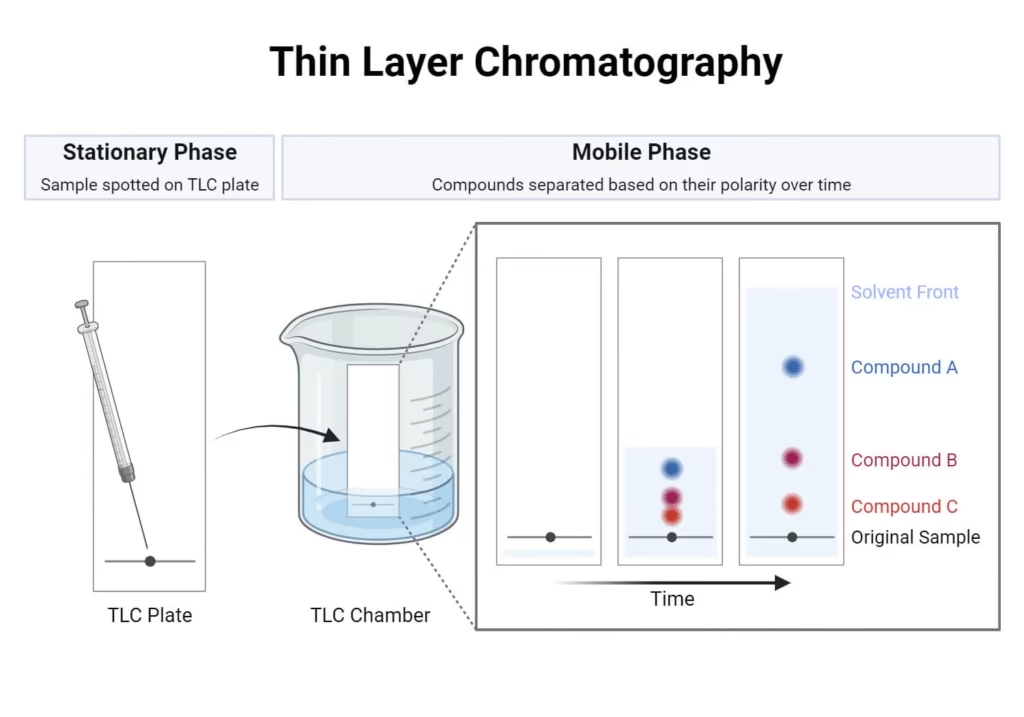
Thin Layer Chromatography (TLC):
- Stationary phase: thin layer of silica gel or alumina on a glass or plastic plate.
- Faster and gives better resolution than paper chromatography.
- Commonly used to check the purity of compounds in organic chemistry.
- Stationary phase is packed inside a column.
- Components separate as they pass through with the mobile phase.
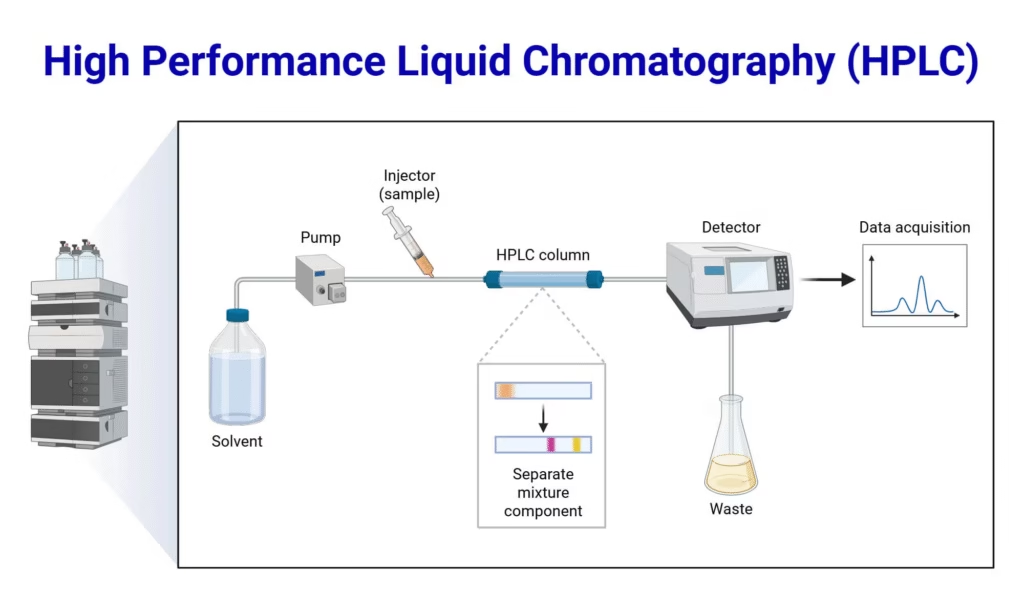
- Examples: Gas Chromatography (GC), High-Performance Liquid Chromatography (HPLC).
B. Based on Separation Mechanism
- Adsorption Chromatography
- Separation based on how strongly molecules stick (adsorb) to the stationary phase.

2. Partition Chromatography
- Separation based on solubility in stationary vs. mobile phase.
- Measured using a partition coefficient.
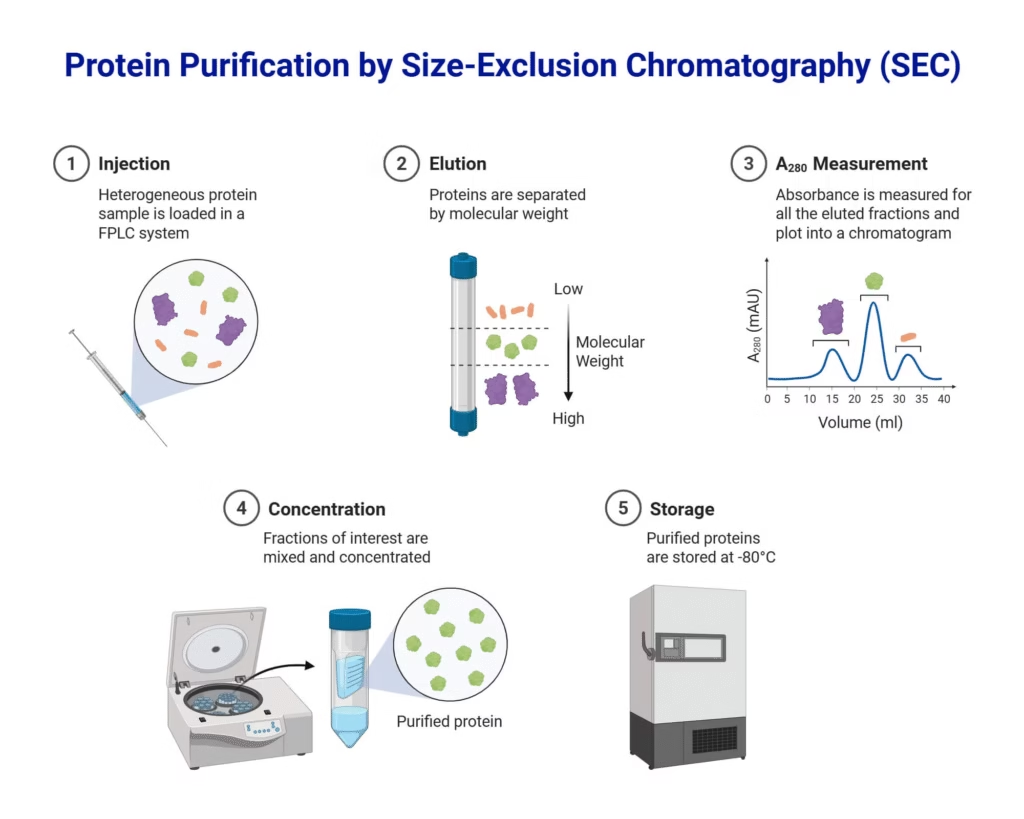
3. Size-Exclusion Chromatography (SEC)
- Separation based on molecular size.
- Large molecules move faster, smaller ones slower (as they enter pores in the stationary phase).
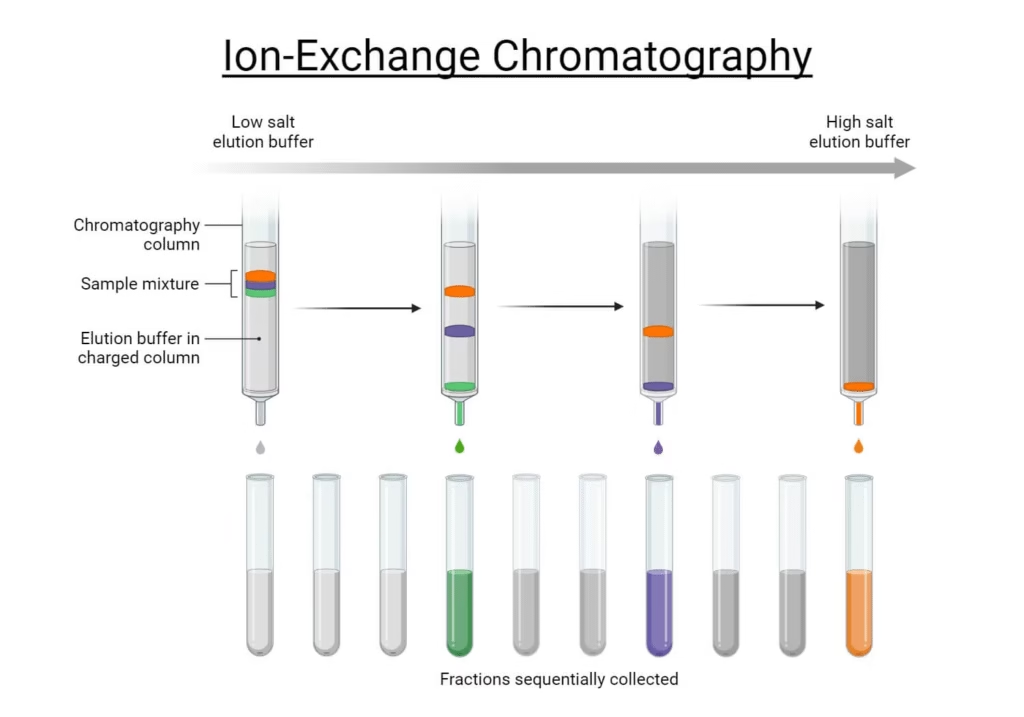
3. Ion Exchange Chromatography (IEC)
- Separation based on charge of molecules.
- Cation exchange: stationary phase has negative charges (retains positively charged molecules).
- Anion exchange: stationary phase has positive charges (retains negatively charged molecules).
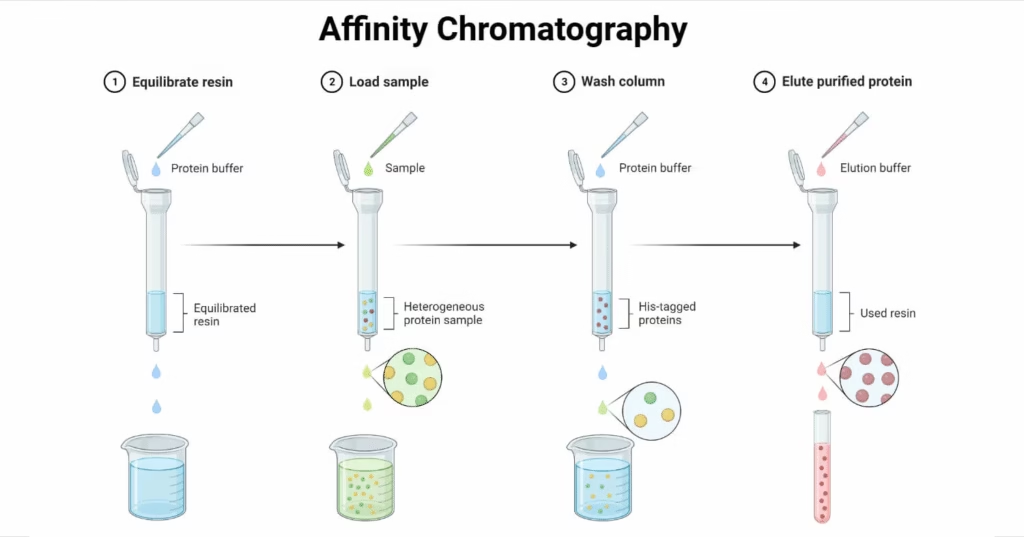
- Uses specific biological interactions (antigen-antibody, enzyme-substrate) to isolate a target molecule.
- Highly selective, often used for protein purification.
C. Based on Phases
- Gas Chromatography (GC)
- Mobile phase: gas (helium, nitrogen, or hydrogen).
- Used for volatile compounds, environmental testing, perfume analysis.
- Liquid Chromatography (LC)
- Mobile phase: liquid.
- Includes HPLC, which uses high pressure and specialized columns for fast, accurate separation.
Steps in a Chromatography Process
- Sample Preparation
- Remove impurities via filtration, centrifugation, or extraction.
- Phase Selection
- Choose stationary and mobile phases based on sample properties.
- Sample Application
- Introduce sample into the system (spotting on TLC plate or injecting into HPLC/GC).
- Separation
- Mobile phase carries sample components at different rates through the stationary phase.
- Detection and Analysis
- Detectors record results as chromatograms.
- Peak position → compound identity.
- Peak height/area → concentration.
Factors Affecting Chromatographic Separation
- Sample properties (size, polarity, charge)
- Mobile phase composition, pH, temperature
- Stationary phase material and particle size
- Flow rate (high flow = faster but less separation; low flow = better separation)
Applications of Chromatography
- Environmental Testing – Monitor water and air quality, detect pollutants.
- Forensic Science – Identify drugs, toxins, and biological samples.
- Pharmaceutical Industry – Drug development, purity testing, quality control.
- Food Industry – Detect additives, contaminants, and verify nutritional content.
- Bioprocessing – Purify vaccines, enzymes, and monoclonal antibodies.
- Chemical Industry – Test purity and properties of chemicals.
Advantages of Chromatography
- Works with complex mixtures.
- High accuracy and sensitivity (detects tiny amounts).
- Wide applicability (small molecules → large biomolecules).
- Can be paired with mass spectrometry for advanced analysis.
Limitations of Chromatography
- High cost of equipment (HPLC, GC).
- Requires trained operators.
- Sample preparation can be time-consuming.
- Compounds with very similar properties can be difficult to separate.
Recent Innovations on Chromatography
- Portable chromatography devices for field testing.
- Nanoparticle-based stationary phases for better efficiency.
- Integration with AI for retention time prediction and data analysis.
- Green chromatography using eco-friendly solvents and less waste.
Therefore, Chromatography is a powerful, versatile tool in biology, chemistry, medicine, and industry.
It provides accurate separation and analysis of mixtures, making it essential for research, diagnostics, and quality control. With ongoing innovations, chromatography continues to become faster, more accurate, and more eco-friendly.
References
- Coskun O. (2016). Separation techniques: Chromatography. Northern clinics of Istanbul, 3(2), 156–160. https://doi.org/10.14744/nci.2016.32757
- Debnath, S., Das, M., Mondal, S., Sarkar, B. K., & Babu, G. (2025). Advances in chromatography: contemporary techniques and applications. Essential Chem, 2(1), 1–27. https://doi.org/10.1080/28378083.2025.2466624
- Factors affecting chromatographic separation | Solubility of things. (n.d.). Retrieved from https://www.solubilityofthings.com/factors-affecting-chromatographic-separation
- Keller, A, R., Giddings, & Calvin, J. (2025, April 8). Chromatography | Definition, Types, & Facts. Retrieved from https://www.britannica.com/science/chromatography/Subsequent-developments
- Kumar, P. (2015, October 16). Top 12 types of chromatographic techniques | Biochemistry. Retrieved from https://www.biologydiscussion.com/biochemistry/chromatography-techniques/top-12-types-of-chromatographic-techniques-biochemistry/12730
- Pan, S. (2025, February 9). Chromatography – principle, types, applications – Biology Notes online. Retrieved from https://biologynotesonline.com/chromatography-principle-types-applications/
- Premnath, S. M., & Zubair, M. (2024, January 11). Chromatography. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK599545/s
Other related topics you might be interested in:
Adsorption Chromatography – Principle, Types, Procedure, Applications & Advantages
Affinity Chromatography – Principle, Components, Procedure, Applications & Advantages
Gas Chromatography (GC) – Principle, Parts, Procedure, Steps, Applications, Advantages & Limitations
High-Performance Liquid Chromatography (HPLC) – Principle, Instrumentation, Types & Applications
Ion Exchange Chromatography – Principle, Instrumentation, Procedure, Applications
Paper Chromatography – Definition, Principle, Types, Steps, Applications, Advantages & Limitations
Thin Layer Chromatography (TLC) – Principle, Steps, Applications, Advantages & Limitations