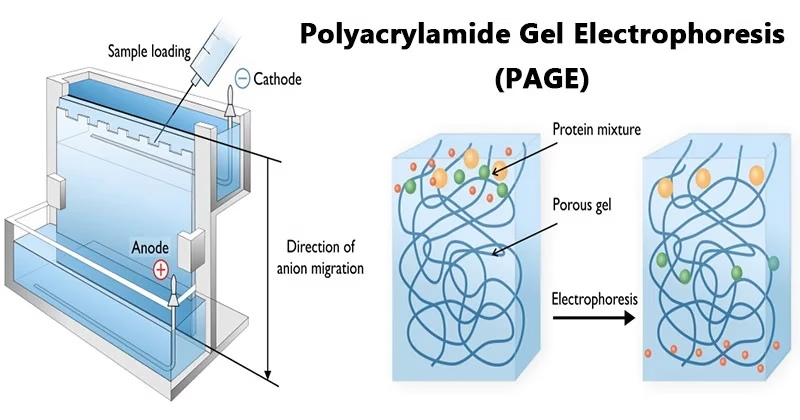
Introduction to Polyacrylamide Gel Electrophoresis
- Electrophoresis is a standard method used in molecular biology and biochemistry to separate, identify, and analyze biomolecules like proteins and nucleic acids.
- The separation is achieved by forcing molecules to migrate through a porous gel matrix under an electric field.
- Two main gel types are used: agarose gel and polyacrylamide gel.
- Polyacrylamide Gel Electrophoresis (PAGE) provides greater resolution than agarose gels, especially for proteins and small DNA fragments.
- PAGE is widely used in molecular biology, genetics, forensic science, biochemistry, and biotechnology.
In simple terms: PAGE is a laboratory technique used to separate proteins and nucleic acids based on their size and charge by making them move through a polyacrylamide gel under electricity.
What is Polyacrylamide Gel?
- Polyacrylamide gels are chemically cross-linked gels formed by the polymerization of acrylamide with a cross-linking agent such as N,N’-methylenebisacrylamide.
- The polymerization is initiated by ammonium persulfate (APS) and catalyzed by TEMED (N,N,N’,N’-tetramethylethylenediamine).
- The pore size of the gel depends on the ratio of acrylamide to bisacrylamide, making it highly adjustable for separating molecules of different sizes.
- This makes polyacrylamide gels superior to agarose gels when working with small proteins or DNA fragments.
Principle of PAGE
The principle of PAGE is based on two main concepts:
- Electrophoretic Mobility
- Charged molecules migrate in an electric field towards the electrode with the opposite charge.
- The rate of migration depends on charge, size, and shape of the molecule.
- Denaturation for Size-Based Separation
- Different proteins naturally have different shapes and charges, which makes comparison difficult.
- To overcome this, proteins are treated with SDS (Sodium Dodecyl Sulfate), which:
- Denatures proteins (removes secondary, tertiary, quaternary structures).
- Covers them with a uniform negative charge.
- Thus, the separation depends only on size (molecular weight).
In summary: In SDS-PAGE, proteins move through the gel at different speeds based on their molecular size, with smaller proteins migrating faster.
Requirements for PAGE
To perform PAGE, the following materials are required:
- Acrylamide and Bisacrylamide solution (for resolving and stacking gels).
- Gel loading buffer (contains tracking dye and denaturants).
- Running buffer (commonly Tris-Glycine buffer).
- Staining and destaining solutions (Coomassie Brilliant Blue, silver stain, or ethidium bromide for nucleic acids).
- Protein or nucleic acid samples.
- Molecular weight markers (protein ladders) for comparison.
- Electrophoresis equipment:
- Gel casting stand and glass plates
- Combs for sample wells
- Electrophoresis chamber and power supply
Steps in Polyacrylamide Gel Electrophoresis (PAGE)
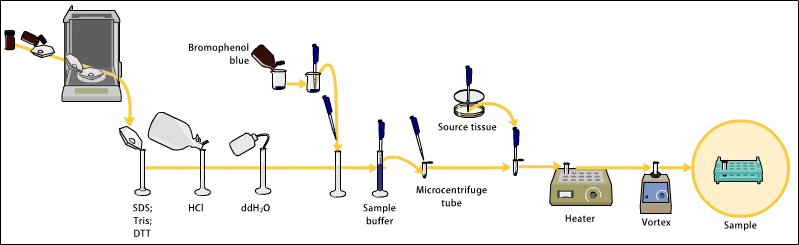
1. Sample Preparation
- Protein samples are mixed with SDS to denature and impart uniform negative charge.
- A reducing agent (like β-mercaptoethanol or DTT) may be added to break disulfide bonds.
- Samples are heated (~60–100°C) to ensure full denaturation.
- Tracking dye is added to monitor electrophoresis progress.
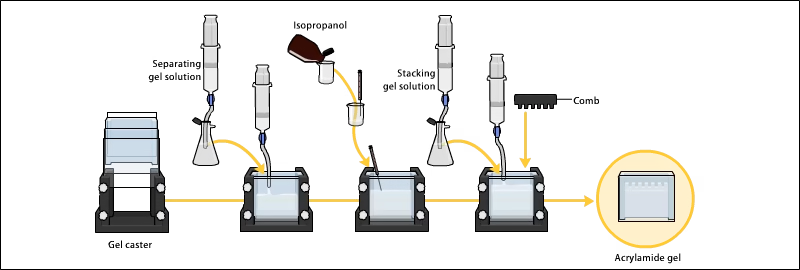
2. Preparation of Polyacrylamide Gel
- Gel consists of acrylamide + bisacrylamide, APS, TEMED, and buffer.
- Two layers are prepared:
- Resolving gel: Higher % acrylamide (5–25%) for separating proteins.
- Stacking gel: Lower % acrylamide to concentrate samples before separation.
- Gel is cast between two glass plates and wells are created with combs.
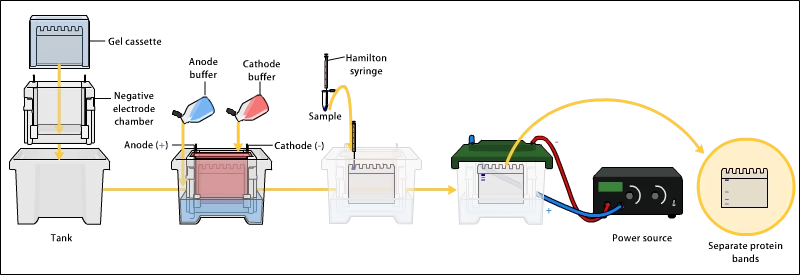
3. Loading Samples and Running the Gel
- After polymerization, wells are filled with samples and a molecular weight marker.
- The gel is placed in the electrophoresis chamber filled with buffer.
- Electric current is applied: negatively charged molecules migrate towards the anode.
- Smaller proteins migrate faster through the pores, larger ones slower.
4. Detection/Visualization
- After electrophoresis, gels are stained to visualize separated molecules.
- Common stains:
- Coomassie Brilliant Blue (routine protein staining).
- Silver stain (high sensitivity).
- Ethidium bromide or SYBR Green (for nucleic acids).
- After staining, bands appear at different positions corresponding to proteins/DNA of different sizes.
Applications of PAGE
- Protein Analysis
- Estimation of molecular weight.
- Determination of protein purity.
- Study of protein subunits and quaternary structures.
- Peptide mapping.
- Nucleic Acid Studies
- Analysis of small DNA or RNA fragments.
- Detection of mutations or polymorphisms.
- Medical and Clinical Uses
- Diagnosing protein abnormalities.
- Analyzing hemoglobin variants.
- Studying enzyme deficiencies.
- Research and Biotechnology
- Used before Western blotting.
- Quality control in recombinant protein production.
- Comparing protein composition between samples.
- Forensic Applications
- Identification of proteins/DNA in criminal investigations.
Advantages of PAGE
- High resolving power – sharp, distinct bands.
- Adjustable pore size by changing acrylamide concentration.
- Can separate proteins of similar size effectively.
- Produces highly pure DNA or protein fragments.
- Works well for low molecular weight proteins.
Disadvantages of PAGE
- Preparation is more complex than agarose gels.
- Acrylamide is toxic, requiring careful handling.
- Gels are less reusable – a new gel must be prepared for each run.
- More time-consuming and prone to technical errors.
Types of PAGE
- SDS-PAGE (Denaturing PAGE)
- Most common type.
- Proteins denatured with SDS → separation based only on size.
- Native PAGE (Non-denaturing PAGE)
- Proteins retain native structure and activity.
- Separation depends on charge, shape, and size.
- 2D-PAGE (Two-dimensional PAGE)
- Combines isoelectric focusing (IEF) and SDS-PAGE.
- Used for complex proteomic studies.
Conclusion
- Polyacrylamide Gel Electrophoresis (PAGE) is one of the most important molecular biology techniques for analyzing proteins and nucleic acids.
- With variations like SDS-PAGE, Native PAGE, and 2D-PAGE, it plays a crucial role in diagnostics, proteomics, clinical studies, and biotechnology research.
- Despite being labor-intensive and involving toxic chemicals, its accuracy and resolution make PAGE a gold standard in protein analysis.
Frequently Asked Questions (FAQs) on Polyacrylamide Gel Electrophoresis (PAGE)
Q1. What is Polyacrylamide Gel Electrophoresis (PAGE)?
Ans: PAGE is a laboratory technique used to separate proteins and nucleic acids by moving them through a polyacrylamide gel under an electric field.
Q2. Why is polyacrylamide used instead of agarose?
Ans: Polyacrylamide has smaller, adjustable pores, giving higher resolution for separating proteins and small DNA fragments compared to agarose.
Q3. Who introduced PAGE?
Ans: The technique was developed in the 1950s and 1960s by scientists studying protein separation, later refined into SDS-PAGE by Ulrich K. Laemmli in 1970.
Q4. What is the principle of PAGE?
Ans: Molecules migrate in an electric field at different speeds depending on charge, size, and shape. In SDS-PAGE, separation is based mainly on molecular weight.
Q5. What are the types of PAGE?
Ans:
- SDS-PAGE (denaturing) → size-based separation.
- Native PAGE → separation based on charge, size, and shape (proteins remain active).
- 2D-PAGE → combines isoelectric focusing (pH separation) with SDS-PAGE.
Q6. What is SDS-PAGE?
Ans: SDS-PAGE uses sodium dodecyl sulfate (SDS) to denature proteins and give them a uniform negative charge, so migration depends only on size.
Q7. What is Native PAGE?
Ans: In Native PAGE, proteins are not denatured. They retain their structure and activity, and separation depends on charge + size + shape.
Q8. What is the role of the stacking gel in PAGE?
Ans: The stacking gel has low acrylamide concentration and helps concentrate all proteins into sharp bands before entering the resolving gel.
Q9. What is the function of TEMED in PAGE?
Ans: TEMED (Tetramethylethylenediamine) catalyzes the polymerization of acrylamide into polyacrylamide gel.
Q10. Why is acrylamide considered hazardous?
Ans: Acrylamide monomers are toxic and neurotoxic; they must be handled with gloves and safety precautions.
Q11. What stains are used to visualize proteins in PAGE?
Ans: Common stains:
- Coomassie Brilliant Blue (routine staining)
- Silver stain (high sensitivity)
- SYBR Green / Ethidium Bromide (for nucleic acids)
Q12. Can PAGE be used for DNA and RNA?
Ans: Yes. PAGE can separate small DNA and RNA fragments, but agarose gels are preferred for large DNA fragments.
Q13. What is the difference between PAGE and agarose gel electrophoresis?
Ans: PAGE → high resolution, best for proteins & small DNA.
Agarose gel → best for large DNA fragments (hundreds of bp to kb).
Q14. What is the role of SDS in PAGE?
Ans: SDS denatures proteins and binds uniformly, giving all proteins a negative charge proportional to their length, making separation purely size-based.
Q15. What is 2D-PAGE?
Ans: Two-dimensional PAGE first separates proteins by isoelectric focusing (based on charge) and then by SDS-PAGE (based on size). Used in proteomics.
Q16. How is PAGE used in medicine?
Ans: PAGE is used to detect abnormal blood proteins, hemoglobin variants, and enzyme deficiencies, aiding in disease diagnosis.
Q17. How is PAGE used in biotechnology?
Ans: PAGE helps in protein purification, Western blotting, recombinant protein analysis, and quality control.
Q18. What are the advantages of PAGE?
Ans:
- High resolution.
- Adjustable pore size.
- Sharp bands for accurate analysis.
- Useful for low molecular weight proteins.
Q19. What are the disadvantages of PAGE?
Ans:
- Complex preparation compared to agarose.
- Acrylamide is toxic.
- Gel is single-use.
- Time-consuming.
Q20. Why is PAGE important in research?
Ans: PAGE is a fundamental technique in molecular biology for analyzing proteins, nucleic acids, and is essential for proteomics, clinical studies, and biotechnology.
Q21. What is a protein ladder in PAGE?
Ans: A protein ladder (molecular weight marker) is a mixture of proteins of known sizes used to estimate the size of unknown proteins in a sample.
Q22. Can PAGE be quantitative?
Ans: Mostly qualitative/semi-quantitative. However, with densitometry analysis, PAGE results can provide quantitative information.
Q23. What is the difference between SDS-PAGE and Western blotting?
Ans: SDS-PAGE separates proteins by size.
Western blotting transfers separated proteins to a membrane and uses antibodies for specific protein detection.
References
- http://elte.prompt.hu/sites/default/files/tananyagok/IntroductionToPracticalBiochemistry/ch07s03.html
- https://www.wou.edu/las/physci/ch462/Gel%20Electrophoresis.pdf
- https://microbenotes.com/polyacrylamide-gel-electrophoresis-page/
- https://www.slideshare.net/mbn1994/introduction-principle-instrumentation-and-applications-of-sdspage-55728195
- https://en.wikipedia.org/wiki/Polyacrylamide_gel_electrophoresis
- https://msu.edu/course/css/451/Lecture/PT-electrophoresis%20(2009).pdf
- http://library.umac.mo/ebooks/b28050459.pdf
Other related topics you might be interested in:
Agarose Gel Electrophoresis – Principle, Procedure, Applications, Advantages & Limitations