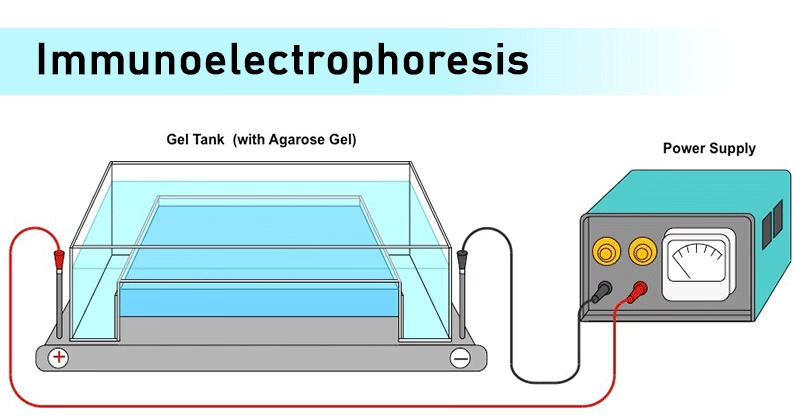
Introduction to Immunoelectrophoresis
- Immunoelectrophoresis (IEP) is a powerful analytical technique that combines the principles of electrophoresis and immunodiffusion.
- In simple terms, it helps to separate and identify proteins (antigens) in a mixture by allowing them to migrate in an electric field and then testing them with specific antibodies.
- The process results in the formation of precipitin lines (arcs) that indicate antigen–antibody interactions.
- This method was first described in 1953 by Grabar and Williams, and it created a breakthrough in protein identification and immunology research.
In short: Immunoelectrophoresis is a protein separation + identification test used in clinical diagnosis, research, and immunology labs.
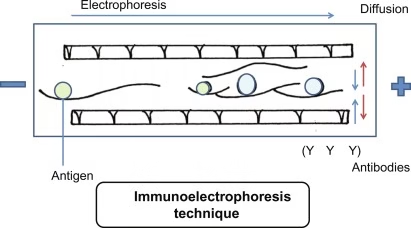
Principle of Immunoelectrophoresis
The principle can be understood in two main steps:
- Electrophoresis
- Proteins (antigens) are placed in a gel (usually agarose) and separated using an electric current.
- The separation is based on charge, size, and mobility of proteins.
- Immunodiffusion
- A trough containing specific antibodies (antisera) is cut parallel to the separated proteins.
- Antigens and antibodies diffuse towards each other.
- Where they meet at optimal proportions, a precipitin line (arc) is formed.
Each arc corresponds to an antigen–antibody reaction, helping to identify different proteins present in the mixture.
Step-by-Step Procedure of Immunoelectrophoresis
- Preparation of Agarose Gel
- A thin layer of agarose gel is prepared on a glass slide in horizontal position.
- Application of Samples
- Small wells are created in the gel using a template.
- Antigen samples are diluted (commonly in a 2:3 ratio with diluent).
- Using a micropipette, a few microliters of the antigen sample and control are applied into the wells.
- Electrophoresis
- The slide is placed in an electrophoresis chamber.
- Samples are kept on the cathodic side, and current is applied (commonly 100 volts for ~20 minutes).
- Proteins migrate based on charge and size.
- Addition of Antisera
- A trough is cut in the gel, parallel to the migration path.
- Specific antisera (antibody solution) is added into the trough.
- Diffusion & Incubation
- The slide is placed in a moist chamber to prevent drying.
- Incubated at room temperature for 18–24 hours.
- Antigens and antibodies diffuse towards each other.
- Washing and Drying
- Gel is washed with saline to remove unbound proteins.
- Dried using blotter sheets and heated below 70°C.
- Staining (Optional)
- Gel may be stained with protein staining solution to highlight arcs.
- Destained to remove background staining.
- Observation of Results
- Elliptical arcs indicate antigen–antibody interaction.
- Absence of arcs = no reaction.
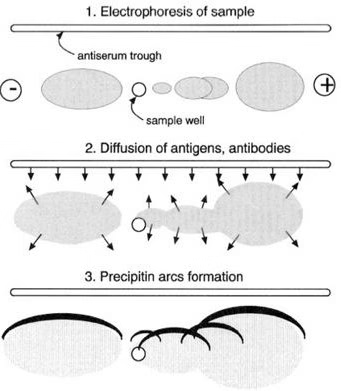
Results of Immunoelectrophoresis
- Positive Result: Visible precipitin arcs form between antigen and antibody wells.
- Negative Result: No arcs observed, meaning no antigen–antibody interaction.
- The intensity, position, and shape of arcs help in identifying and characterizing proteins.
- Multiple arcs suggest multiple antigens in the mixture.
Applications of Immunoelectrophoresis
Immunoelectrophoresis has diverse applications in diagnosis, research, and quality control:
1. Clinical Diagnosis
- Detects abnormal proteins in blood or urine (e.g., myeloma proteins).
- Used in patients with suspected monoclonal or polyclonal gammopathies.
- Helps diagnose conditions like multiple myeloma, Waldenström’s macroglobulinemia, and hypogammaglobulinemia.
2. Protein Analysis
- Identifies normal and abnormal proteins in serum.
- Separates proteins in complex antigen mixtures.
- Monitors antigen and antibody purity.
3. Immunology Research
- Helps understand antigen–antibody interactions.
- Identifies specific proteins in a mixture.
- Useful in vaccine development and immunological studies.
4. Food and Environmental Testing
- Limited applications in food analysis (due to antibody availability).
- Can detect specific proteins in biological or environmental samples.
Advantages of Immunoelectrophoresis
- Combines electrophoresis (separation) with immunodiffusion (specificity).
- High resolving power – can identify multiple proteins simultaneously.
- Useful for protein identification and characterization.
- Helps detect both absence and overproduction of proteins.
- Provides visual evidence of antigen–antibody interactions.
Limitations of Immunoelectrophoresis
- Slower and less sensitive compared to immunofixation electrophoresis.
- Difficult to interpret in complex samples.
- May miss small monoclonal proteins due to overlapping arcs.
- Limited use in food analysis (antibody unavailability).
- Requires 18–24 hours incubation, making it time-consuming.
Conclusion
- Immunoelectrophoresis is a classical but still relevant technique in immunology and clinical diagnostics.
- It combines electrophoresis for protein separation and immunodiffusion for identification.
- Although newer techniques like immunofixation and ELISA are more sensitive, immunoelectrophoresis remains a valuable tool in serum protein analysis, gammopathy diagnosis, and immunology research.
In short: Immunoelectrophoresis is a window into the immune system’s protein world, helping scientists and doctors detect and analyze protein abnormalities.
Frequently Asked Questions (FAQs) on Immunoelectrophoresis
Q1. What is immunoelectrophoresis?
Ans: Immunoelectrophoresis (IEP) is a laboratory technique that combines electrophoresis (protein separation) with immunodiffusion (antigen–antibody interaction) to identify and analyze proteins.
Q2. Who discovered immunoelectrophoresis?
Ans: It was first described by Grabar and Williams in 1953.
Q3. What is the principle of immunoelectrophoresis?
Ans: Proteins are separated in a gel by electrophoresis, and then antibodies diffuse into the gel. Where antigen and antibody meet at optimal proportions, precipitin arcs form, indicating antigen–antibody complexes.
Q4. What type of gel is used in immunoelectrophoresis?
Ans: Usually agarose gel is used because it is transparent, non-toxic, and allows efficient antigen–antibody diffusion.
Q5. What are precipitin lines in immunoelectrophoresis?
Ans: Precipitin lines (arcs) are visible bands formed by antigen–antibody complexes in the gel, confirming protein identification.
Q6. How long does immunoelectrophoresis take?
Ans: Typically 18–24 hours, as antigen and antibody diffusion is a slow process.
Q7. What is the purpose of immunoelectrophoresis?
Ans: To separate, identify, and characterize proteins, and to diagnose diseases related to abnormal protein production.
Q8. What are the applications of immunoelectrophoresis in medicine?
Ans: It is used to detect:
- Multiple myeloma
- Waldenström’s macroglobulinemia
- Gammopathies (mono- and polyclonal)
- Hypogammaglobulinemia and immune deficiencies
Q9. What is the difference between immunoelectrophoresis and immunofixation electrophoresis?
Ans:
- Immunoelectrophoresis: Older, less sensitive, requires long incubation.
- Immunofixation: Faster, more sensitive, better for detecting small monoclonal proteins.
Q10. Is immunoelectrophoresis still used today?
Ans: Yes. It is still used in some clinical labs, though modern techniques like immunofixation and ELISA have become more common.
Q11. What are the advantages of immunoelectrophoresis?
Ans:
- Identifies multiple proteins simultaneously.
- Simple and visual method.
- Useful for detecting protein abnormalities in serum.
Q12. What are the limitations of immunoelectrophoresis?
Ans:
- Less sensitive compared to modern techniques.
- Overlapping arcs may cause interpretation difficulties.
- Requires long incubation (overnight).
Q13. Can immunoelectrophoresis detect monoclonal proteins?
Ans: Yes. But it is less sensitive compared to immunofixation electrophoresis, which is preferred for small monoclonal proteins.
Q14. What kind of samples are used in immunoelectrophoresis?
Ans: Commonly serum and urine samples are used to study protein composition.
Q15. What does a positive result in immunoelectrophoresis mean?
Ans: Presence of precipitin arcs indicates antigen–antibody interactions, confirming the presence of specific proteins.
Q16. What does a negative result mean in immunoelectrophoresis?
Ans: Absence of arcs suggests that no antigen–antibody reaction occurred, meaning the target protein is absent.
Q17. What conditions can immunoelectrophoresis help diagnose?
Ans: Diseases like multiple myeloma, Waldenström’s macroglobulinemia, immune deficiencies, gammopathies, and other protein abnormalities.
Q18. How is immunoelectrophoresis different from simple electrophoresis?
Ans: Simple electrophoresis only separates proteins, while immunoelectrophoresis also identifies proteins using antibodies.
Q19. Is immunoelectrophoresis qualitative or quantitative?
Ans: It is primarily a qualitative or semi-quantitative test, not fully quantitative like spectrophotometric methods.
Q20. Can immunoelectrophoresis be automated?
Ans: No. It is largely a manual process, though modern diagnostic labs often replace it with automated methods like immunofixation or ELISA.
Q21. Why is immunoelectrophoresis important in immunology?
Ans: Because it helps visualize specific antigen–antibody reactions, which is crucial for studying immune function and disorders.
Q22. What are the modern alternatives to immunoelectrophoresis?
Ans: Immunofixation electrophoresis, ELISA, Western blotting, and capillary electrophoresis are faster and more sensitive.
References
- Lydyard, P.M., Whelan,A.,& Fanger,M.W. (2005).Immunology (2 ed.).London: BIOS Scientific Publishers.
- Parija S.C. (2012). Textbook of Microbiology & Immunology.(2 ed.). India: Elsevier India.
- http://www.hellabio.com/E89B86BA.en.aspx
- Actor, J.K. (2014). Assessment of Immune Parameters and Immunodiagnostics. Introductory Immunology. Pages 135-152
- https://microbenotes.com/immunoelectrophoresis-principle-procedure-results-and-applications-advantages-and-limitations/
- Sastry A.S. & Bhat S.K. (2016). Essentials of Medical Microbiology. New Delhi : Jaypee Brothers Medical Publishers.
Other related topics you might be interested in:
Agarose Gel Electrophoresis – Principle, Procedure, Applications, Advantages & Limitations