What are Vaccines?
- Vaccine (Latin: vacca = cow) is a preparation/suspension or extract of dead/attenuated (weakened) germs of a disease which, on inoculation (injection) into a healthy person, provides temporary/permanent active/passive immunity by inducing antibody formation.
- The process of introducing a vaccine into an individual to provide protection against a disease is called vaccination.
Types of Vaccines
Vaccine types can broadly be classified into three groups:
- Whole-organism vaccines
- Live-attenuated vaccines
- Inactivated vaccines
- Subunit vaccines
- Polysaccharide vaccines
- Conjugated vaccines
- Toxoid vaccines
- Nucleic acid vaccines
- DNA plasmid vaccines
- mRNA vaccines
- Recombinant vector vaccines
[I] Whole-organism vaccines
- Many vaccines that were developed early consist of an entire pathogen that is either killed (inactivated) or weakened (attenuated) so that they cannot cause disease. These are known as whole-organism vaccines.
- These vaccines elicit strong protective immune responses, and many vaccines used today are prepared in this manner. However, not all disease-causing microbes can be effectively targeted with a whole-organism vaccine.
a) Live-attenuated vaccines
- These vaccines are prepared from a whole organism by weakening their pathogenicity so that they do not cause disease but can induce an immune response, hence the term attenuation.
- These vaccines elicit strong immune responses because they are similar to the actual disease pathogen. Hence, they confer life-long immunity after only one or two doses. Therefore, they are very effective.
b) Inactivated (killed) vaccines
- These are produced by killing the pathogen (bacteria, virus, or other pathogens) with chemicals, heat, or radiation.
- The killed pathogen cannot cause disease, which means they do not replicate in the host’s body.
[II] Subunit vaccines
- These are vaccines that are prepared by using components or antigens of the pathogen. These components can stimulate the immune system to elicit appropriate immune responses.
- They are also known as acellular vaccines because they do not contain a whole cell but just parts of a cell of bacteria or virus.
a) Polysaccharide vaccines
- Some microbes contain a polysaccharide (sugar) capsule which they use for protection and evading the human immune defenses, especially in infants and young children.
- Thus, polysaccharide vaccines are those vaccines that are prepared using the sugar molecules, and polysaccharides from the outer layer of a bacteria or virus.
b) Conjugated vaccines
- These vaccines are prepared by linking the polysaccharides or sugar molecules on the outer layer of the bacteria to a carrier protein antigen or toxoid from the same microbe.
- The linkage of polysaccharides with a protein greatly increases the ability of the immune systems of young children to recognize the polysaccharide and develop immunity.
c) Toxoid vaccines
- These vaccines are prepared from inactivated toxins by treating the toxins with formalin, a solution of formaldehyde and sterilized water.
- The process of inactivation of toxins is known as detoxification and the resultant toxin is known as toxoid.
- The toxins used for the preparation of toxoids are obtained from the bacteria that secrete illness-causing toxins.
[III] Nucleic acid vaccines
- Nucleic acid vaccines use genetic material from a disease-causing virus or bacterium (a pathogen) to stimulate an immune response against it.
- Once inserted into host cells, this genetic material is read by the cell’s own protein-making machinery and used to manufacture antigens, which then trigger an immune response.
Some of the known nucleic acid vaccine models include:
a) DNA plasmid vaccines
- These are vaccines that are composed of a small circular piece of DNA known as a plasmid. The plasmid carries genes that encode proteins from the pathogen of interest.
- Experimental DNA plasmid vaccines have been designed by the National Institute of Allergy and Infectious Disease (NIAID) to address some viral disease threats including SARS coronavirus (SARS-CoV) in 2003, H5N1 avian influenza in 2005, H1N1 pandemic influenza in 2009, and Zika virus in 2016.
b) mRNA vaccines
- mRNA is an intermediary between DNA and protein. Recent technological advances have developed mRNA vaccines overcoming the instability issues of mRNA and its delivery into the cells, with encouraging results.
- Some experimental mRNA vaccines have been designed to protect mice and monkeys against Zika virus infection, and administered in a single dose.
c) Recombinant vector vaccines
- These are vaccines designed as vectors or carriers using harmless viruses or bacterium which introduce the genetic material into cells.
- Majorly these vaccines are designed and approved for use to protect animals from infectious diseases, including rabies and distemper, but some have also been developed to protect humans from viruses such as HIV, Zika virus, and Ebola virus.
Production of Vaccines
Steps in vaccine production
Upstream processing
→ Selecting the strains for vaccine production
→ Growing the microorganisms
Downstream processing
→ Isolation and purification of microorganism
→ Inactivation of microorganism
→ Formulation of vaccine
→ Quality control and lot release
1. Selecting the strains for vaccine production
- The Seed (strain)
- Manufacturing begins with small amount of a specific virus or a specific bacteria (called seed)
- Viruses or Bacteria used in manufacture shall be derived from a Seed Lot System.
- A record of the origin, passage history (including purification and characterization procedures) and storage conditions should be maintained for each seed lot.
- The virus must be free of impurities, including other similar viruses and even variations of the same type of virus.
- The seed must be kept under “ideal” conditions, usually frozen, that prevents the virus from becoming either stronger or weaker than desired.
- Selecting the seed (strain)
- The choice of the seed depends on a number of factors including the efficacy of the resulting vaccine and its secondary effects.
- If possible, the bacterial strain or cell line should be obtained from a recognised culture collection with an established and documented provenance.
- Alternatively, if the chosen vaccine strain is an “in house” clinical isolate, it will be necessary to compile a complete history of the strain, including details of its isolation, identification, and maintenance for product registration.
2. Growing the microorganisms
Growing bacteria
Methods used are:
- Batch culture ⇒ The microbe is grown in a closed vessel, typically in a test tube or flask.
- Continuous culture ⇒ The microbe is grown in vessel which has medium constantly added, and spent medium constantly removed. It is performed in a chemostat.
Growing viruses
Methods used are:
- Cell (tissue) cultures ⇒ Cultured cells grow in sheets that support viral replication and permit observation for cytopathic effect.
- Bird embryos ⇒ Incubating egg is an ideal system; virus is injected through the shell.
- Live animal inoculation ⇒ used when necessary.
- Transgenic animals
3. Isolation and Purification of microorganism
- Product isolation is the removal of those components whose properties vary markedly from that of the desired product.
- Purification selectively separates and retains the desired product at the highest purity as per its pre-determined specification.
- This can be achieved by using following techniques:
- Centrifugation
- Chromatography
- Filtration
- The most common method of vaccine production is based on an initial fermentation process followed by purification.
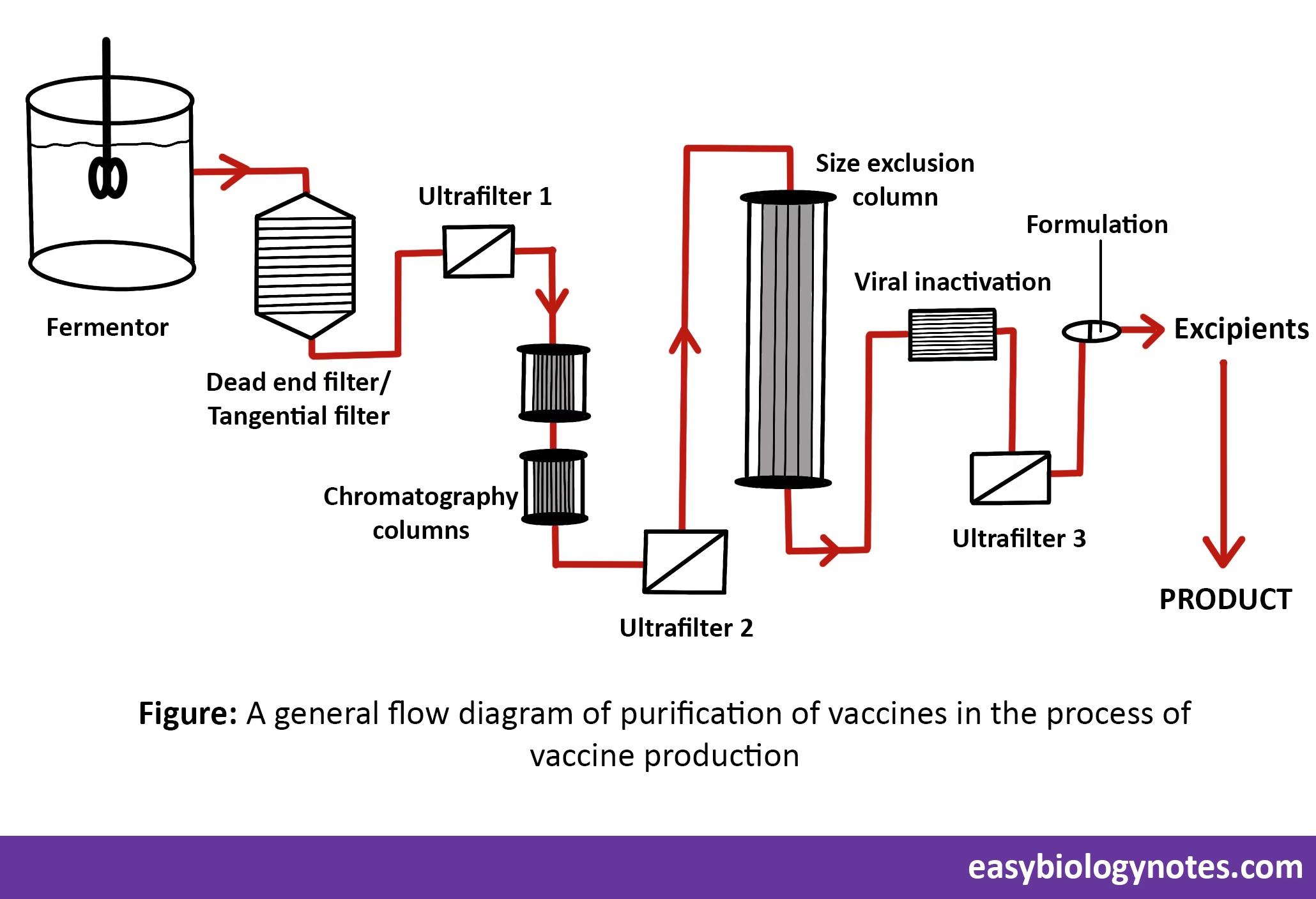
4. Inactivation of Microorganism
Virus inactivation
- Viruses can be lipid – coated (enveloped) or non enveloped.
- Virus inactivation involves dismantling a virus’s ability to infect cells without actually eliminating the virus.
- Virus inactivation works by one of the following two mechanisms:
- By attacking the viral envelope or capsid and destroying its ability to infect or interact with cells.
- By disrupting the viral DNA or RNA preventing replication.
- Virus inactivation can be achieved by following techniques:
- Solvent/detergent (S/D) inactivation
- Pasteurization
- Acidic pH inactivation (Low pH treatment)
- Ultraviolet (UV) inactivation
Bacterial inactivation can be achieved by extraction of nucleic acid, inclusion bodies, membrane extraction, capsule extraction.
5. Formulation of Vaccine
Other than microorganism or its part, a vaccine contain the following substances:
a) Suspending fluids – The liquid which contains the chemicals used during production which kill or weaken the organism for use in vaccines. Examples: Sterile water, saline water, or fluids containing protein (such as Egg proteins or Yeast Proteins)
b) Preservatives and stabilizers (the vaccine remain unchanged)
Examples:
- Albumin, Phenols, Glycine
- Monosodium glutamate (MSG) and 2-phenoxy-ethanol which are used as stabilizers in a few vaccines to help the vaccine remain unchanged when the vaccine is exposed to heat, light, acidity, or humidity.
- Antibiotics, which are added to some vaccines to prevent the growth of bacteria during production and storage of the vaccine, eg, neomycin, streptomycin, polymyxin B, etc.
- Thimerosal is a mercury-containing preservative that is added to vials of vaccine that contain more than one dose to prevent contamination & growth of potentially harmful bacteria
c) Inactivating agents
- Formaldehyde is used to inactivate bacterial products for toxoid vaccines. It is also used to kill unwanted viruses and bacteria that might contaminate the vaccine during production! Used in influenza virus, poliovirus, etc.
- Glutaraldehyde is used to inactivate toxins contained in acellular pertussis vaccines.
- β-propiolactone is used to inactivate rabies virus.
d) Adjuvants or enhancers – these enhance vaccine immunogenicity. Eg., Aluminium gels or salts (alum).
6. Quality control and lot release
Several tests are performed before final product & manufacturing. These tests are:
- Sterility test
- Chemistry
- Safety
- Residual toxicity
- Efficacy
- Increase in virulence tests
- Assessing risk to the environment
- Evaluation of the consistency of production
- Stability tests
Lot release: Prior to release, the manufacturer must test each batch/serial for purity, safety and potency.
Labelling: Standards for labelling products will vary from country to country.