Introduction
- Chromatography is a laboratory technique used to separate components of a mixture based on their interactions with a stationary phase (fixed) and a mobile phase (moving).
- Affinity chromatography is a specialized type of liquid chromatography that uses specific and reversible biological interactions between the target molecule and a ligand attached to the stationary phase.
- Discovered in 1968 by Pedro Cuatrecasas and Meir Wilchek.
- Falls under adsorption chromatography, but is much more specific and selective.
- Commonly used to purify proteins, enzymes, antibodies, and other biomolecules.
Principle of Affinity Chromatography
- Based on the lock-and-key mechanism:
- Lock = ligand attached to stationary phase.
- Key = specific site on the target molecule that binds to the ligand.
- The target molecule binds specifically and reversibly to the ligand, while non-target molecules pass through.
- Binding is due to:
- Hydrogen bonds
- Electrostatic forces
- Hydrophobic interactions
- Van der Waals forces
- Elution (release of bound molecules) is done by:
- Adding a competing ligand to the mobile phase
- Changing pH, ionic strength, or polarity
Key Features
- Highly selective – isolates only the desired molecule from a mixture.
- Can be single-step (fast, high purity) or multi-step (for complex purification strategies).
- Works best when a specific ligand for the target molecule is available.
Components of Affinity Chromatography
1. Matrix
- Inert solid support that holds the ligand.
- Must be:
- Chemically and physically stable
- Insoluble in solvents and buffers
- Have a large surface area for maximum ligand attachment
- Good flow properties for smooth column operation
- Examples: agarose beads, polyacrylamide beads, polystyrene, cellulose.
2. Spacer Arm
- Short chemical link that connects ligand to matrix.
- Prevents steric hindrance (crowding) so the target molecule can bind easily.
- Examples: 1,6-diaminohexane, 6-aminohexanoic acid.
3. Ligand
- Molecule that binds specifically to the target molecule.
- Can be:
- Biological (e.g., antibodies, enzymes, glycoproteins)
- Synthetic (e.g., biomimetic dyes, metal chelates, boronates)
- Selection of ligand depends on target molecule:
- Antigen → Antibody isolation
- Substrate/inhibitor → Enzyme isolation
- Metal ions → Histidine-tagged proteins
- Types:
- Monospecific ligands – bind to one or few molecules (e.g., enzyme inhibitors, vitamins).
- Group-specific ligands – bind to a broad class of molecules (e.g., boronic acid derivatives, biomimetic dyes).
Examples of Ligand-Target Pairs
| Ligand | Target Molecule |
| Antigen | Antibody |
| Substrate | Enzyme |
| Lectin | Glycoprotein, polysaccharide |
| Complementary DNA sequence | Nucleic acids |
| Metal ions | His-tagged proteins |
| Protein A or G | Immunoglobulins |
| Phenyl boronate | Glycoproteins |
| Poly(A) | Poly(U) RNA |
Types of Affinity Chromatography
1. Boronate & Phenyl Borate Affinity
- Uses boronate ligands.
- Commonly used for glycoprotein purification and HbA1c (glycated hemoglobin) analysis.
2. Lectin Affinity
- Uses lectins (carbohydrate-binding proteins) as ligands.
- Separates polysaccharides, glycopeptides, and carbohydrate-containing cells.
3. Dye-Ligand Affinity
- Uses synthetic dyes as ligands.
- Purifies blood proteins, enzymes, and pharmaceuticals.
4. Immunoaffinity
- Uses antibodies as ligands to purify hormones, viruses, peptides, and enzymes.
5. Immobilized Metal Ion Affinity Chromatography (IMAC)
- Uses immobilized metal ions (e.g., Ni²⁺, Co²⁺) to bind proteins with histidine residues.
- Widely used for recombinant His-tagged protein purification.
6. Analytical Affinity Chromatography
- Used for quantitative analysis and measurement of target molecules.
Sample Preparation
- Remove particulates using filtration or centrifugation.
- Buffer should be optimized for:
- pH
- Ionic strength
- Compatibility with ligand-target binding.
- Avoid components that disrupt interactions (e.g., detergents, denaturing agents).
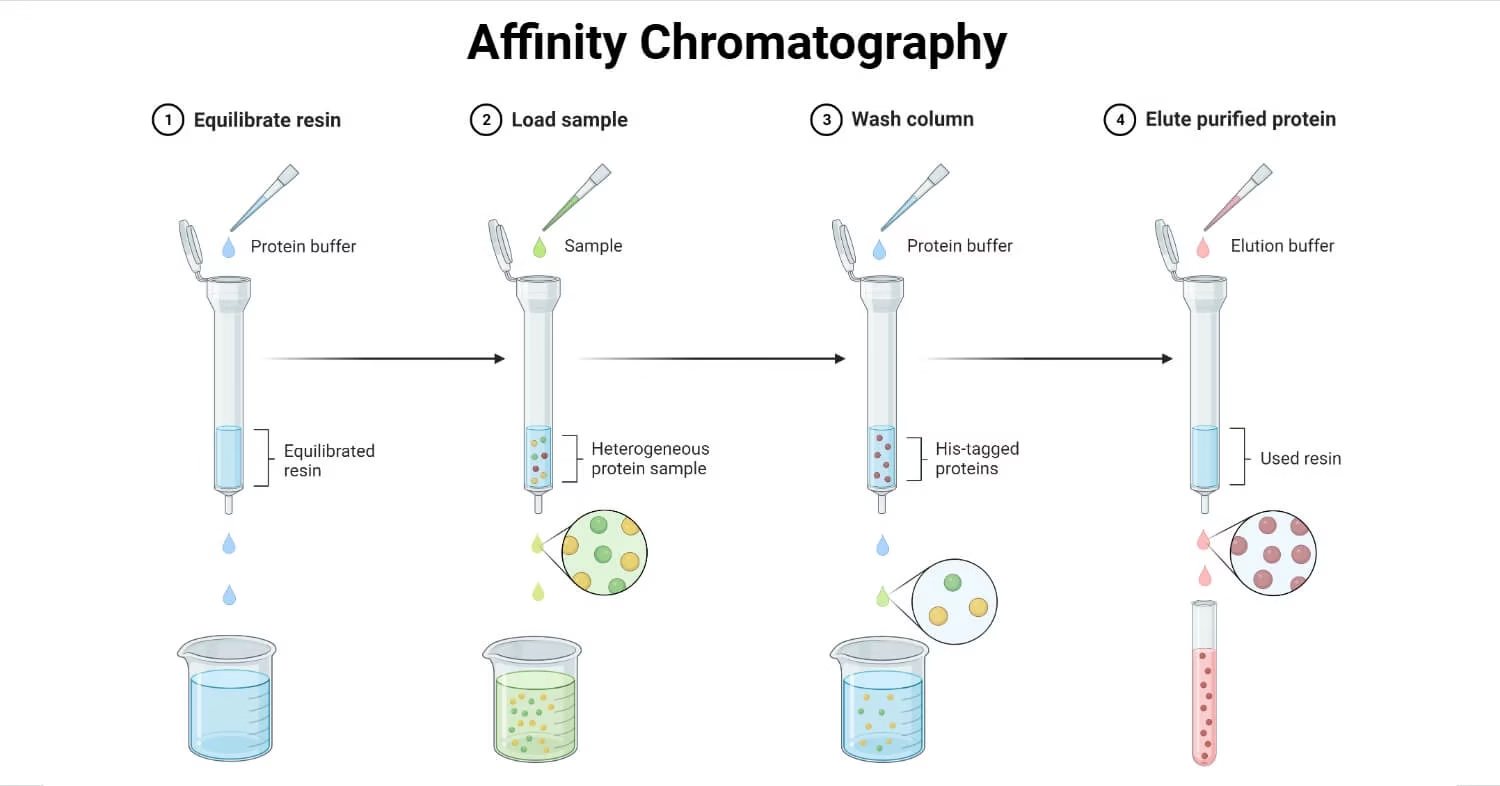
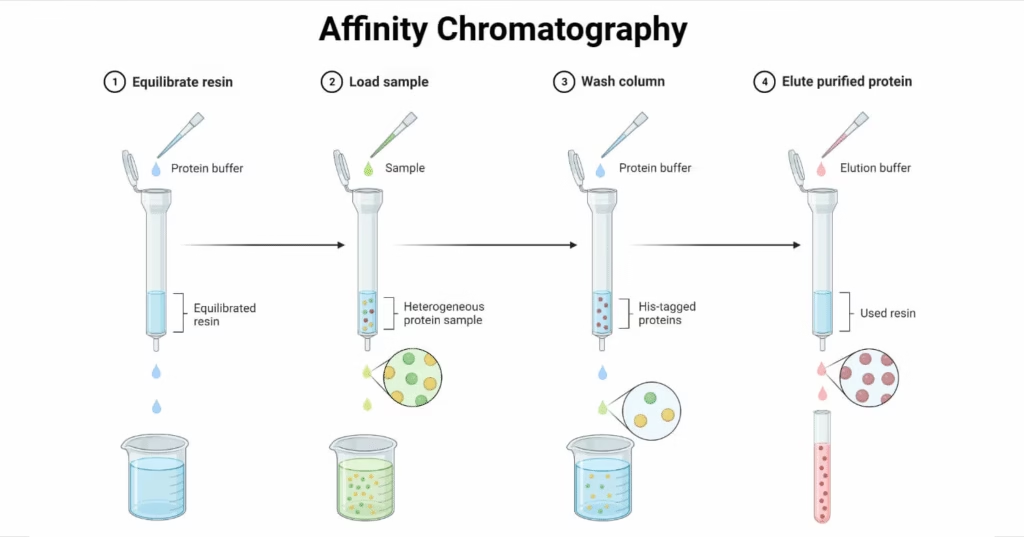
Procedure – Steps in Affinity Chromatography
- Column Preparation
- Pack column with chosen matrix.
- Attach ligand to matrix via spacer arm.
- Equilibrate column with binding buffer.
- Sample Loading
- Apply sample under conditions that favor binding between target molecule and ligand.
- Non-target molecules pass through.
- Washing
- Remove weakly bound or non-specific molecules using wash buffer.
- Elution
- Release target molecules using:
- Competitive ligand
- pH change
- Ionic strength change
- Chaotropic agents
- Release target molecules using:
- Re-equilibration
- Prepare column for reuse by washing with buffer.
Factors Affecting Efficiency
- Specificity of Ligand – must match target molecule.
- Binding Strength – too weak → loss of target; too strong → hard to elute.
- Matrix Properties – high surface area and appropriate pore size.
- pH & Buffer Composition – maintain stability of ligand and target.
- Flow Rate – slower rates improve binding but increase time.
Applications of Affinity Chromatography
- Purification of enzymes, proteins, antibodies.
- Isolation of active biomolecules from crude extracts.
- Separation of functional proteins from denatured forms.
- Detection of biomarkers for disease diagnosis.
- Purification of monoclonal antibodies in pharmaceutical manufacturing.
Advantages of Affinity Chromatography
- Extremely specific – isolates only target molecule.
- Produces highly pure products in a single step.
- Can process large volumes.
- Matrix is reusable.
Limitations of Affinity Chromatography
- Ligands can be expensive and may not be available for all targets.
- Time-consuming preparation.
- Non-specific binding can occur.
- Sensitive to pH changes – risk of denaturing proteins.
Troubleshooting Tips
- Use fresh, filtered samples to avoid clogging.
- Degas buffers to remove air bubbles.
- Store columns in 20% ethanol to prevent microbial growth.
- Avoid extreme pH unless required for elution.
Recent Innovations
- Use of magnetic beads and monolithic supports for faster separation.
- Development of nanobody-based ligands for higher specificity.
- Miniaturized, automated chromatography systems for precise control.
Affinity chromatography is a powerful, highly selective technique for purifying biomolecules.
Its ability to exploit specific ligand-target interactions makes it a key method in biochemistry, biotechnology, and pharmaceutical industries.
With advancements in ligands and automated systems, it continues to become faster, more efficient, and more widely applicable.
References
- Labrou, N. (2003). Design and selection of ligands for affinity chromatography. Journal of Chromatography B, 790(1–2), 67–78. https://doi.org/10.1016/s1570-0232(03)00098-9
- Introduction to affinity Chromatography. (n.d.). Bio-Rad Laboratories. https://www.bio-rad.com/en-np/applications-technologies/introduction-affinity-chromatography?ID=LUSMJIDN
- Hage, D. S., Anguizola, J. A., Bi, C., Li, R., Matsuda, R., Papastavros, E., Pfaunmiller, E., Vargas, J., & Zheng, X. (2012). Pharmaceutical and biomedical applications of affinity chromatography: Recent trends and developments. Journal of Pharmaceutical and Biomedical Analysis, 69, 93–105. https://doi.org/10.1016/j.jpba.2012.01.004
- Banjara, M.R. and Thapa Shrestha, U. (2021). Instrumentation in Microbiology. Garuda Publications.
- Wilson, K. and Walker, J. (Ed.). (2010). Principles and Techniques of Biochemistry and Molecular Biology. Seventh Edition. Cambridge University Press.
- Jason, J. C. (Ed.). (2011). Protein Purification: Principle, High-Resolution Methods, and Applications. Third Edition. John Wiley & Sons Inc.
- GE Healthcare. A handbook on Affinity Chromatography: Principles and Methods.
- Pan, S. (2025, February 10). Affinity chromatography – Principle, Types, Steps, Applications – Biology Notes Online. Biologynotesonline.com. https://biologynotesonline.com/affinity-chromatography-principle-types-steps-applications/
Other related topics you might be interested in:
Chromatography – Principle, Types, Steps, Uses, and Advantages
Adsorption Chromatography – Principle, Types, Procedure, Applications & Advantages
Gas Chromatography (GC) – Principle, Parts, Procedure, Steps, Applications, Advantages & Limitations
High-Performance Liquid Chromatography (HPLC) – Principle, Instrumentation, Types & Applications
Ion Exchange Chromatography – Principle, Instrumentation, Procedure, Applications
Paper Chromatography – Definition, Principle, Types, Steps, Applications, Advantages & Limitations
Thin Layer Chromatography (TLC) – Principle, Steps, Applications, Advantages & Limitations