What is Fluorimetry?
- Fluorimetry is an analytical technique used to measure the fluorescent light emitted by a substance when it absorbs ultraviolet (UV) or visible light.
- It helps in studying the concentration, properties, and interactions of compounds in a sample.
- The emitted light always has a longer wavelength (lower energy) compared to the absorbed light. This phenomenon is known as luminescence or “cold light”.
In simple terms: Fluorimetry tells us how a substance glows under UV light, and this glow helps scientists study its properties.
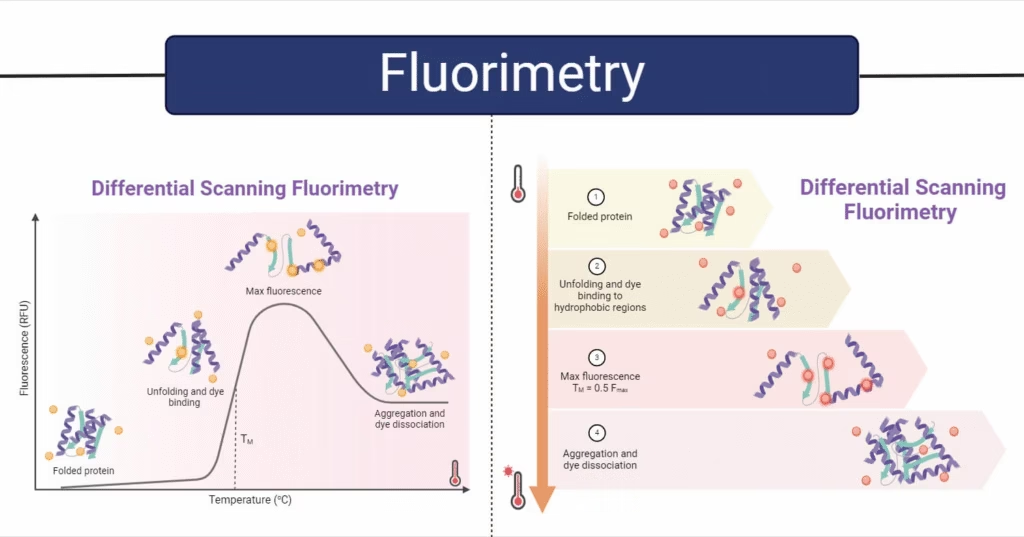
Luminescence and Its Types
Luminescence = Light emission at low temperature, without producing heat.
1. Fluorescence
- Instantaneous emission of light.
- Starts immediately after absorption of light.
- Stops as soon as the light source is removed.
- Example: Glow of fluorescent dyes, GFP (Green Fluorescent Protein).
2. Phosphorescence
- Also called delayed fluorescence.
- Light emission continues even after the source is removed.
- Example: Glow-in-the-dark toys.
Principle of Fluorimetry
- Fluorimetry works on the principle of fluorescence emission.
- When light of a specific wavelength strikes a molecule, it excites the electrons to a higher energy state.
- Since this excited state is unstable, the electrons quickly return to their normal (ground) state.
- During this process, energy is released as photons of visible light.
- The emitted light has less energy and longer wavelength than the absorbed light.
The device used to measure this fluorescence is called a Fluorimeter.
Three possible fates of an excited electron:
- Deactivation without emission → Energy lost as heat (no light).
- Fluorescence → Immediate emission of visible light.
- Phosphorescence → Delayed emission of visible light.
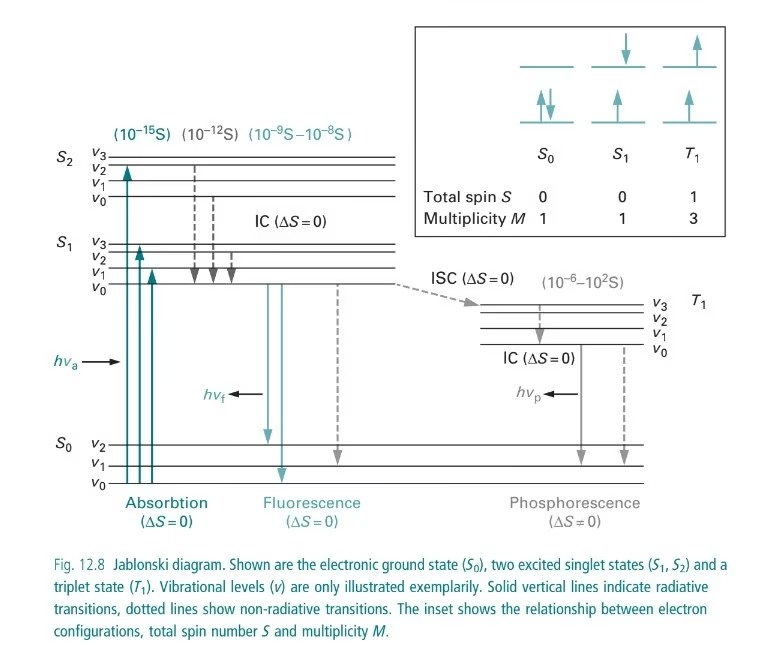
Jablonski Diagram in Fluorescence
- A Jablonski diagram shows energy transitions in a molecule.
- Steps include:
- Excitation (absorption of UV/visible light).
- Vibrational relaxation (energy lost as heat).
- Emission of fluorescence or phosphorescence.
Components of Fluorimeter
A fluorimeter has several key components:
1. Light Source
- Provides excitation energy.
- Types:
- Mercury vapor lamp → High intensity, commonly used.
- Xenon arc lamp → More intense, used in spectrofluorometers.
- Hydrogen/Deuterium lamp → Useful for UV range (160–800 nm).
- Tungsten (halogen) lamp → Low-cost instruments.
2. Filters & Monochromators
- Primary filter/monochromator → selects UV radiation for excitation.
- Secondary filter/monochromator → isolates emitted fluorescence (blocks UV, transmits visible light).
3. Sample Holder (Cuvettes)
- Holds the sample.
- Made of quartz, silica, or glass.
- Designed so that emitted light is detected at a right angle to the incident beam (to avoid interference).
4. Detector
- Converts light into electrical signals.
- Common types:
- Photovoltaic/Barrier cell – simple, low sensitivity.
- Photoemissive tube – more sensitive, uses photocathode.
- Photomultiplier tube (PMT) – highly sensitive; detects even very weak fluorescence.
5. Readout System
- Displays fluorescence intensity.
- Can be analog (galvanometer) or digital (modern fluorimeters with software integration).
Types of Spectra in Fluorimetry
- Excitation Spectrum → Intensity of fluorescence measured while varying excitation wavelength.
- Emission Spectrum → Intensity measured at different emission wavelengths.
Fluorophores and Their Role
Fluorophores = Molecules that absorb light and re-emit fluorescence.
Types:
- Organic Dyes → Fluorescein, Rhodamine, AMCA.
- Biological Fluorophores → GFP (Green Fluorescent Protein), chlorophyll, tryptophan.
- Quantum Dots → Nanocrystals, highly stable, long-lasting fluorescence.
Factors Affecting Fluorescence
- Molecular Structure
- Rigid molecules → more fluorescence.
- Flexible molecules → less fluorescence.
- Solvent Nature
- Polar solvents → increase fluorescence.
- Viscous solvents → reduce collisions, increase fluorescence.
- Temperature
- High temperature → decreases fluorescence (more collisions).
- Low temperature → increases fluorescence.
- Concentration of Fluorophores
- Works best at low concentrations (<0.02 absorbance).
- Very high concentrations cause “self-quenching”.
- Intensity of Incident Light
- Stronger excitation light → stronger fluorescence.
- Quenching
- Reduction of fluorescence due to other molecules.
- Types:
- Collision quenching (collisions with halides, oxygen).
- Static quenching (complex formation).
Applications of Fluorimetry
In Biological Sciences
- Protein & Nucleic Acid Quantification (DNA, RNA, enzymes).
- Characterization of biomolecules (conformation, stability).
- Biological Imaging (fluorescent microscopy, GFP tagging).
In Environmental Science
- Detection of pollutants, pesticides, heavy metals in water/soil.
- Analysis of smoke, exhaust gases, and industrial effluents.
In Medicine & Pharmaceuticals
- Drug discovery & screening (binding studies, enzyme activity).
- Vitamin detection (B1, B2 in food).
- Cancer diagnosis (fluorescence-based tumor detection).
In Nuclear Research
- Measurement of uranium salts and radioactive tracers.
In Industrial & Chemical Research
- Study of metals in alloys (chromium, manganese, aluminum).
- Analysis of synthetic polymers and nanomaterials.
Advantages of Fluorimetry
- Highly sensitive (detects trace quantities).
- Precise and accurate quantification.
- Relatively low cost and fast technique.
- Non-destructive for samples.
- Can measure fluorescence decay time.
Limitations of Fluorimetry
- Only works with fluorescent substances.
- Fluorophores have a short lifespan.
- Sensitive to temperature, pH, and solvent changes.
- Quenching effects may interfere with accuracy.
Precautions in Fluorimetry
- Use clean cuvettes (no scratches or dust).
- Avoid bubbles in the sample.
- Use proper filters/monochromators for accurate wavelength selection.
- Protect fluorophores from light exposure (they degrade easily).
- Calibrate instrument regularly.
FAQs on Fluorimetry
Q1. What is the difference between fluorescence and phosphorescence?
Ans: Fluorescence stops immediately after light is removed, phosphorescence continues.
Q2. Why is fluorimetry more sensitive than colorimetry?
Ans: Because fluorescence can be detected at very low concentrations.
Q3. What are fluorophores?
Ans: Molecules that absorb light at one wavelength and emit at a longer wavelength.
Q4. Can fluorimetry be used in medical diagnosis?
Ans: Yes, it is used in cancer detection, vitamin analysis, and drug studies.
Q5. What is quenching in fluorimetry?
Ans: Reduction of fluorescence due to other molecules (oxygen, halides, impurities).
Conclusion
Fluorimetry is an important analytical technique that helps detect and quantify fluorescent substances with high sensitivity and precision.
From molecular biology and drug discovery to environmental monitoring and cancer diagnosis, it has a wide range of applications.
In short: Fluorimetry allows us to study molecules by observing the light they emit, making it a powerful tool in science and industry.
References
- Fluorimetry. Instrumental Methods of Analysis. Retrieved from https://monad.edu.in/img/media/uploads/IMA(BP-701)%20(U-1P-3)Fluorimentry.pdf. Accessed on 24th January 2024.
- Gao, K., Oerlemans, R., & Groves, M. R. (2020). Theory and applications of differential scanning fluorimetry in early-stage drug discovery. Biophysical reviews, 12(1), 85-104. Retrieved from https://link.springer.com/article/10.1007/s12551-020-00619-2. Accessed on 29th January 2024.
- Karki, G. (2020). Fluorimetry- Principle and Applications. Retrieved from https://www.onlinebiologynotes.com/fluorimetry-principle-and-applications/. Accessed on 26th January 2024.
- What is Fluorimetry? Theory and Application. (2023). Retrieved from https://pharmasiksha.com/fluorimetry-theory-application/. Accessed on 20th January 2023.
- Wilson, K. and Walker, J. (2010). Principles and Techniques of Biochemistry and Molecular Biology. 7th Edition. Cambridge University Press.
Other related topics you might be interested in:
Flow Cytometry – Definition, Principle, Steps, Types, Applications, Advantages & Limitations
Homogenizer – Principle, Parts, Types, Working, Procedure, Applications, Advantages & Limitations
Micropipette – Definition, Types, Parts, Working, Applications, Errors, Calibration & Limitations
Ultracentrifuge – Principle, Types, Parts, Working Procedure, Applications, Advantages & Precautions
Chromatography – Principle, Types, Steps, Uses, and Advantages
Types of Spectroscopy – Principles, Types, Steps, and Applications
