Introduction
A bioreactor is a specialized vessel used for biological reactions and fermentation processes. It provides a controlled environment where microorganisms, plant cells, or animal cells can grow and produce useful products like antibiotics, enzymes, organic acids, and biofuels.
Bioreactors are widely used in biotechnology, pharmaceuticals, agriculture, food industries, and environmental management. They are designed to maintain the right temperature, pH, oxygen level, agitation, and sterility so that cells or microbes can thrive and produce the desired compounds efficiently.
In simple words, a bioreactor acts as a factory for living cells, turning raw materials into valuable biological products.

Principle of Bioreactor
The working of a bioreactor is based on the principle of biotransformation – using living cells or enzymes to convert substrates into desired products.
- Microorganisms (bacteria, fungi, yeast) or cultured cells are grown in the reactor.
- The reactor maintains optimum conditions like temperature, oxygen, and pH.
- Cells use nutrients to grow and produce biomass, metabolites, enzymes, or secondary products.
- Products are then extracted for industrial, medical, or research use.
Essentially, a bioreactor provides the right environment for maximum productivity while ensuring sterility and safety.
Design of a Bioreactor
The design of a bioreactor depends on:
- The type of organism used (bacteria, fungi, algae, mammalian cells).
- The type of product (enzyme, antibiotic, protein, biomass).
- Scale of production (lab, pilot plant, or industrial).
A good design ensures:
- High productivity at low cost.
- Controlled environment for cell growth.
- Easy sterilization and cleaning.
- Long-term durability under pressure and temperature changes.
Construction Material
- Must be non-corrosive and non-toxic.
- Should withstand steam sterilization and high pressure.
- Common materials: stainless steel (for large scale) and glass (for laboratory scale).
Size Range
Bioreactors vary in size:
- Small laboratory fermenters (1–50 L).
- Pilot-scale reactors (0.3–10 m³).
- Industrial plant-scale reactors (2–500 m³).
Parts of a Bioreactor

A modern bioreactor has several parts that ensure proper growth of microorganisms:
1. Fermenter Vessel
- A closed container (cylindrical shape).
- Made of glass (small scale) or stainless steel (large scale).
- Designed to operate under sterile and controlled conditions.
2. Heating and Cooling System
- Maintains optimum temperature.
- Cooling jackets and coils remove excess heat generated during fermentation.
- Thermostats regulate heating when required.
3. Aeration System
- Supplies oxygen in aerobic bioprocesses.
- Uses sparger and impeller to distribute air evenly.
- Ensures proper gas exchange and mixing.
4. Agitation System (Impellers)
- Impellers stir the culture to:
- Distribute nutrients evenly.
- Break air into fine bubbles for better oxygen transfer.
- Prevent cell clumping.
5. Baffles
- Metal strips fixed inside the vessel.
- Prevent swirling (vortex formation) and improve mixing.
6. Sparger
- Introduces sterile air through fine pores (5–10 mm).
- Types: porous sparger, nozzle sparger, combined sparger-agitator.
7. Sealing Assembly
- Prevents contamination and leakage around the stirrer shaft.
- Types: packed gland seal, mechanical seal, magnetic drives.
8. Feed Ports
- Tubes used to add nutrients, acid, or alkali during the process.
- Sterilized before use.
9. Foam Control Unit
- Prevents excess foam which can lead to contamination.
- Uses foam-sensing devices and antifoam agents.
10. Valves
- Control movement of liquid, air, and gases.
- Types: globe valve, butterfly valve, ball valve, diaphragm valve.
11. Environmental Control Devices
- Sensors and monitors for:
- Temperature
- pH
- Oxygen concentration
- Nutrient levels
- Cell density
12. Computer Integration
- Modern bioreactors use automated systems to monitor and control processes in real time.
Types of Bioreactors
1. Continuous Stirred Tank Bioreactor (CSTR)
- Cylindrical vessel with impellers and sparger.
- Most commonly used in industries.
- Advantages: easy operation, cheap construction, simple cleaning.
- Used for: antibiotics, citric acid, tissue culture.

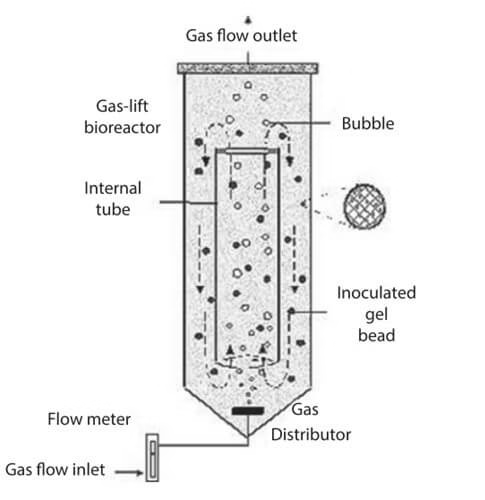
2. Airlift Bioreactor
- Also called tower reactor.
- Divides fluid into two zones: riser (gas sparged) and downcomer (no sparging).
- Advantages: no moving parts, easy sterilization, low energy cost.
- Used for: antibiotics, enzymes, tissue culture.
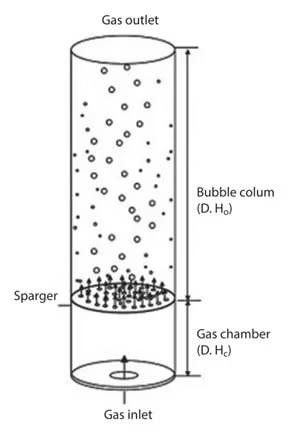
3. Bubble Column Bioreactor
- Cylindrical vessel with gas sparger.
- Mixing occurs through rising gas bubbles.
- Advantages: simple design, easy maintenance, low cost.
- Used for: algal culture, wastewater treatment.
4. Fluidized Bed Bioreactor
- Uses small immobilized particles for enhanced mass transfer.
- Advantages: better oxygen transfer, continuous operation, even temperature.
- Used for: enzyme production, immobilized cell processes.

5. Packed Bed Bioreactor
- Solid particles with immobilized enzymes or cells.
- Advantages: high conversion efficiency, easy operation, low cost.
- Used for: hydrogen production, mammalian cell culture.

6. Photobioreactor
- Transparent unit illuminated by sunlight/artificial light.
- Used for photosynthetic organisms like algae and cyanobacteria.
- Applications: production of pigments (β-carotene), wastewater treatment.

7. Membrane Bioreactor
- Combines traditional bioprocess with membrane filtration.
- Removes suspended solids and nutrients effectively.
- Used for: wastewater treatment, antibiotic production.

Applications of Bioreactors
- Pharmaceutical Industry: production of antibiotics, vaccines, therapeutic proteins.
- Food Industry: production of vitamins, enzymes, amino acids, alcoholic beverages.
- Agriculture: biofertilizers, biopesticides, plant tissue culture.
- Environmental Science: wastewater treatment, bioremediation of polluted soil.
- Bioenergy: production of biofuels like ethanol, hydrogen, and biogas.
- Research: controlled growth of cells for studying metabolism, genetics, and disease models.
Advantages of Bioreactors
- Provide controlled conditions for high yield.
- Scalable for laboratory to industrial production.
- Allow growth of both microorganisms and higher cells.
- Enable production of valuable products at lower cost.
- Help in waste treatment and pollution control.
Limitations of Bioreactors
- High installation and maintenance cost.
- Foaming and contamination risks.
- Some types (e.g., photobioreactors) require complex maintenance.
- Non-uniform mixing in large reactors.
- Membrane bioreactors face biofilm growth issues.
Frequently Asked Questions (FAQ)
Q1. What is a bioreactor in simple words?
It is a fermentation vessel that provides a controlled environment for cells or microbes to produce useful products.
Q2. What are the main types of bioreactors?
Stirred tank, airlift, bubble column, fluidized bed, packed bed, photobioreactor, and membrane bioreactor.
Q3. What are bioreactors used for?
Production of antibiotics, enzymes, vaccines, biofuels, food products, and wastewater treatment.
Q4. Which is the most common type of bioreactor?
The stirred tank bioreactor (CSTR) is most widely used in industries.
Q5. What materials are used to make bioreactors?
Stainless steel (large scale) and glass (small scale).
References
- Chisti, Y. (2006). Bioreactor design. Basic Biotechnology: Third Edition, m, 181–200. https://doi.org/10.1017/CBO9780511802409.009
- Ghosh, B., Bhattacharya, D., & Mukhopadhyay, M. (2018). Use of Fermentation Technology for Value-Added Industrial Research. Principles and Applications of Fermentation Technology, August, 141–161. https://doi.org/10.1002/9781119460381.ch8
- Jaibiba, P., Vignesh, S. N., & Hariharan, S. (2020). Working principle of typical bioreactors. In Bioreactors. INC. https://doi.org/10.1016/B978-0-12-821264-6.00010-3
- Mandenius, C.-F. (2016). Challenges for Bioreactor Design and Operation. Bioreactors, 1–34. https://doi.org/10.1002/9783527683369.ch1
- Muniraj, I. K., Desikan, R., & Subburamu, K. (2019). Perspectives and Prospects of Fermentation Technology. Advances in Food Bioproducts and Bioprocessing Technologies, July 2020, 217–232. https://doi.org/10.1201/9780429331817-10
- Rose, A. H. (1985). Principles of fermentation technology. In Trends in Biotechnology (Vol. 3, Issue 9). https://doi.org/10.1016/0167-7799(85)90016-2
Other related topics you might be interested in:
Autoclave – Principle, Parts, Procedure, Types, Uses, Advantages, Limitations
Bunsen Burner – Principle, Parts, Types, Flames, Applications, Advantages & Precautions
Flow Cytometry – Definition, Principle, Steps, Types, Applications, Advantages & Limitations
Homogenizer – Principle, Parts, Types, Working, Procedure, Applications, Advantages & Limitations
Laboratory Incubator – Principle, Types, Components, Working, Applications, Advantages & Limitations
Laminar Flow Hood / Cabinet – Principle, Types, Parts, Procedure, Applications & Precautions
Micropipette – Definition, Types, Parts, Working, Applications, Errors, Calibration & Limitations
pH Meter – Principle, Parts, Working, Procedure, Types, Applications, Advantages & Precautions
Pipettes – Principle, Types, Uses, Parts, Operation, Advantages & Precautions
Ultracentrifuge – Principle, Types, Parts, Working Procedure, Applications, Advantages & Precautions
Vortex Mixer – Definition, Principle, Parts, Types, Working, Applications, Advantages & Precautions