What is a Colorimeter?
- A colorimeter is a laboratory instrument used in colorimetry to measure how much light a solution absorbs or transmits.
- It helps determine the concentration of colored compounds in a solution by comparing it with a standard reference solution.
- Invented in 1870 by Louis J. Duboscq, it is still widely used in biology, chemistry, clinical labs, food industry, printing, textiles, and environmental monitoring.
In short: A colorimeter tells us how much light is absorbed by a solution and how concentrated it is.
Principle of Colorimeter
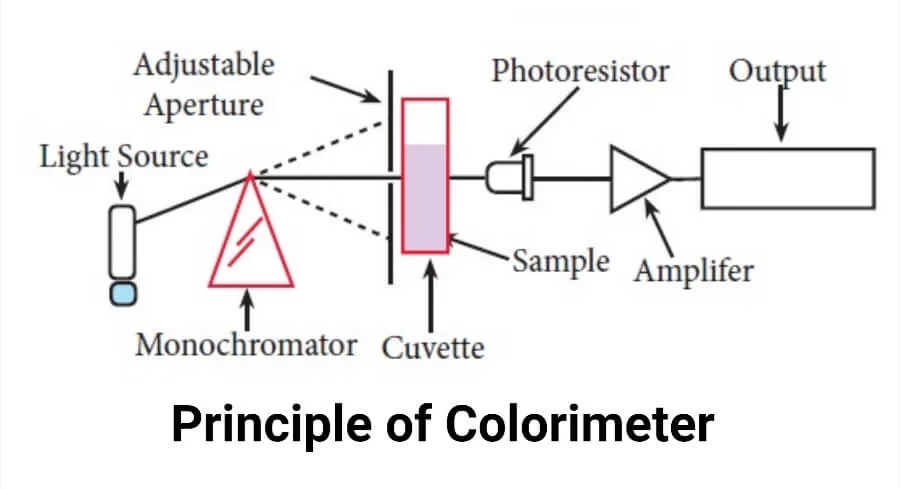
- When a light beam with intensity I₀ passes through a solution, three things happen:
- Some light is reflected (Ir)
- Some is absorbed (Ia)
- Remaining is transmitted (It)
Formula: I₀ = Ir + Ia + It
- The working of a colorimeter is based on the Beer-Lambert Law, which states:
A = εcl
Where,
- A = Absorbance (optical density)
- ε = Molar absorption coefficient
- c = Concentration of the solution
- l = Path length of light through solution
The more concentrated the solution, the more light it absorbs, and the less light passes through.
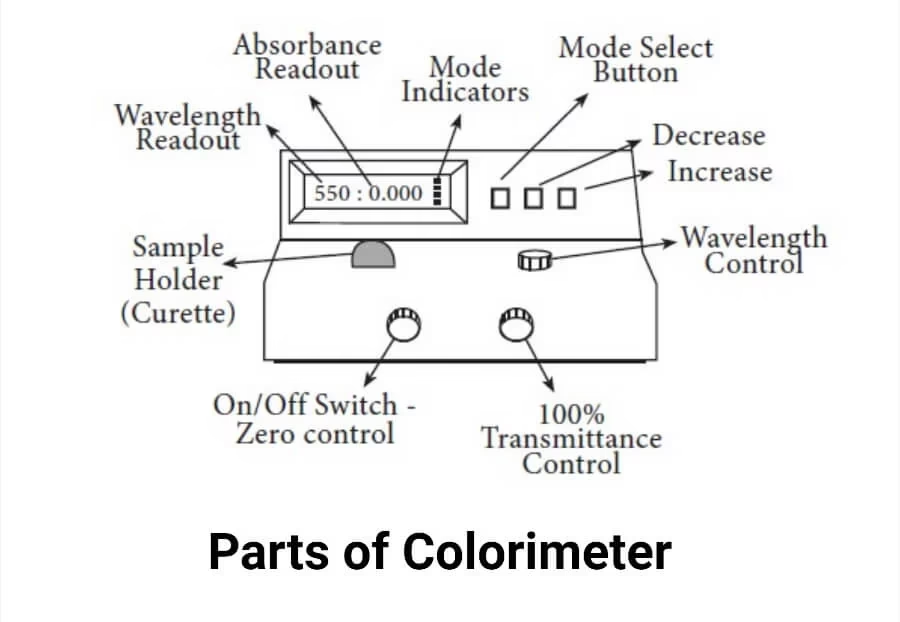
Parts of a Colorimeter
- Light Source – Tungsten lamps (visible light), Halogen/Deuterium (UV range).
- Slit – Allows a narrow beam of light, reduces stray light.
- Condensing Lens – Focuses light into a parallel beam.
- Monochromator (Filter system) – Selects a specific wavelength. Types:
- Prism
- Grating
- Glass filters
- Cuvette (Sample Cell) – Holds the solution. Types:
- Glass (visible light, 340 nm)
- Quartz (UV + visible)
- Plastic (cheap, less durable)
- Photocell (Detector) – Converts light energy into electrical signals.
- Galvanometer/Digital Display – Shows absorbance (A) or % transmittance (T).
All these parts work together to measure how much light is absorbed by the solution.
Types of Colorimeters
- Densitometers – Measure material density.
- Spectrophotometers – Measure spectral transmittance and reflectance.
- Tristimulus Colorimeters – Measure color values using three filters (RGB system).
- Portable Colorimeters – Handheld devices for field testing (e.g., water testing).
Working / Operating Procedure of a Colorimeter
- Switch on the device and let it warm up (15 mins).
- Set the wavelength suitable for your test.
- Calibrate with a blank solution (set 100% transmittance).
- Insert sample solution in cuvette → place in chamber.
- Read absorbance (A) or % Transmittance (T) on display.
- Use calibration curve (Absorbance vs Concentration) to find unknown sample concentration.
Example: Measuring hemoglobin concentration in blood samples.
Applications of Colorimeter
- Clinical labs – Measure hemoglobin, glucose, proteins, enzymes in blood/urine.
- Food & beverage industry – Analyze color quality, flavor compounds, and additives.
- Textile & paint industry – Test dye concentration and pigment quality.
- Pharmaceuticals – Quality testing of drugs.
- Environmental monitoring – Check water purity, detect chemicals (fluoride, iron, chlorine, cyanide).
- Printing industry – Ensure quality of inks and papers.
- Cosmetology – Measure UV protection in skin-care products.
- Gemology – Examine diamonds and gemstones for visual properties.
- Electronics – Test brightness and contrast in TV, mobile, and computer screens.
Advantages of Colorimeter
- Quick and cost-effective.
- Easy quantitative analysis of colored solutions.
- Provides results within seconds.
- Portable devices available.
- Requires very little training to operate.
Limitations of Colorimeter
- Cannot measure colorless solutions directly.
- Works only in visible spectrum (400–700 nm).
- Reflective surfaces may give errors.
- Less accurate than modern spectrophotometers.
- Requires calibration before every test.
Precautions While Using Colorimeter
- Avoid strong external light while taking measurements.
- Use clean cuvettes without scratches or fingerprints.
- Always use same cuvette for blank and another for test sample.
- Do not let dust, liquids, or chemicals enter device.
- Operate in dry, cool environment (avoid humidity).
- Ensure proper ventilation for heat dissipation.
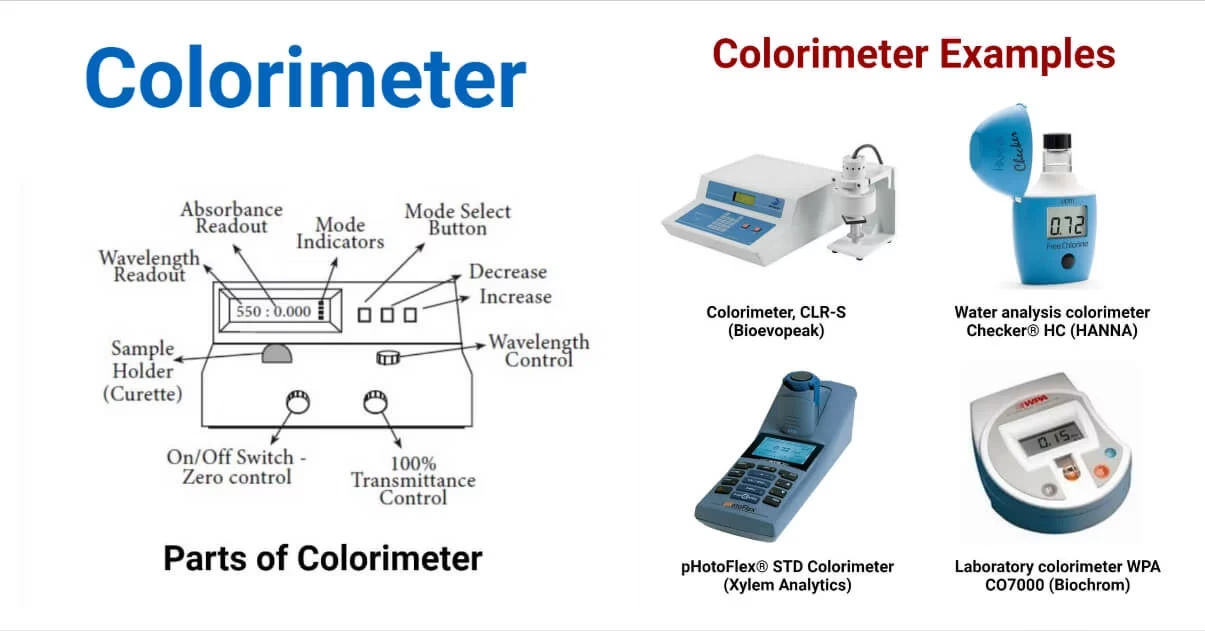
Examples of Colorimeters
- CLR-S (Bioevopeak): Laboratory colorimeter for powders, grains, liquids.
- Checker® HC (HANNA Instruments): Portable water analysis colorimeter.
- pHotoFlex® (Xylem Analytics): Colorimeter with turbidity and pH measurement.
- WPA CO7000 (Biochrom): Portable colorimeter for clinical labs.

FAQs on Colorimeter
Q1. Who invented colorimeter?
Ans: Louis J. Duboscq in 1870.
Q2. What law does colorimeter work on?
Ans: Beer-Lambert Law.
Q3. Can colorimeter measure UV absorption?
Ans: No, only visible range (400–700 nm).
Q4. What is the difference between colorimeter and spectrophotometer?
Ans: Colorimeter uses filters and visible light; spectrophotometer covers UV, visible, IR with higher accuracy.
Conclusion
The colorimeter is a simple yet powerful tool for analyzing colored compounds in solutions. Based on the Beer-Lambert law, it is widely used in biology, medicine, food science, textiles, and environmental analysis.
While it has limitations compared to spectrophotometers, its low cost, portability, and quick results make it indispensable in many labs and industries.
In short: A colorimeter helps scientists, doctors, and industries to measure concentrations of colored solutions accurately and easily.
References
- https://www.ecstuff4u.com/2021/05/advantages-disadvantages-colorimeter.html?m=1
- https://laboratorytests.org/colorimeter/
- https://www.tumblr.com/microamaze/134454969515/precaution-when-using-colorimeter
- https://www.amu.ac.in/department/bio-chemistry-jnmc/sops
- https://www.hunterlab.com/blog/lab-vs-lch-coordinates/
- https://infinitylearn.com/surge/chemistry/colorimeter/
- https://microbenotes.com/colorimeter-definition-principle-parts-uses-examples/
- https://www.medicalexpo.com/prod/bioevopeak/product-301335-1058282.html
- https://www.medicalexpo.com/prod/hanna-instruments/product-80622-863381.html
- https://www.medicalexpo.com/prod/xylem-analytics/product-80700-925763.html
- https://www.medicalexpo.com/prod/biochrom/product-99779-644098.html
Other related topics you might be interested in:
Autoclave – Principle, Parts, Procedure, Types, Uses, Advantages, Limitations
Bioreactor – Principle, Design, Parts, Types, Applications, and Limitations
Bunsen Burner – Principle, Parts, Types, Flames, Applications, Advantages & Precautions
Centrifuge – Principle, Parts, Types, Operation, Applications and Advantages
Colony Counter – Definition, Principle, Types, Parts, Working, Applications, Advantages & Examples
Homogenizer – Principle, Parts, Types, Working, Procedure, Applications, Advantages & Limitations
Hot Air Oven – Principle, Parts, Types, Working, Applications & Advantages
Instruments Used in Microbiology Laboratory – Principles, Uses, and Applications
Laboratory Incubator – Principle, Types, Components, Working, Applications, Advantages & Limitations
Laminar Flow Hood / Cabinet – Principle, Types, Parts, Procedure, Applications & Precautions
Micropipette – Definition, Types, Parts, Working, Applications, Errors, Calibration & Limitations
pH Meter – Principle, Parts, Working, Procedure, Types, Applications, Advantages & Precautions
Pipettes – Principle, Types, Uses, Parts, Operation, Advantages & Precautions
Ultracentrifuge – Principle, Types, Parts, Working Procedure, Applications, Advantages & Precautions
Vortex Mixer – Definition, Principle, Parts, Types, Working, Applications, Advantages & PrecautionsWater Bath – Definition, Principle, Types, Parts, Working, Applications, Advantages, Limitations & Precautions