Introduction to Pulsed Field Gel Electrophoresis
- Pulsed Field Gel Electrophoresis (PFGE) is an advanced laboratory technique used to separate very large DNA molecules.
- Unlike standard gel electrophoresis, PFGE can separate DNA fragments greater than 50 kilobase pairs (kbp) up to several megabase pairs (Mb).
- In conventional agarose gel electrophoresis, DNA fragments larger than ~20 kb move together and form a single band, making it impossible to distinguish them. PFGE solved this limitation.
- The technique was introduced in 1982 by Schwartz and Cantor, who showed that DNA larger than 50 kb could be separated using alternating electric fields.
In simple terms: PFGE is like super gel electrophoresis, designed to handle very large DNA fragments that normal gels cannot separate.

Principle of Pulsed Field Gel Electrophoresis
- Normally, small DNA fragments can easily move through agarose gel pores, but large DNA fragments (>30–50 kb) migrate at the same rate, forming a diffuse band.
- PFGE applies an electric field that periodically changes direction.
- During each pulse:
- Smaller DNA fragments realign quickly and move faster.
- Larger DNA fragments take longer to reorient and lag behind.
- Over time, this difference allows fragments of varying sizes (from a few kb to >10 Mb) to separate into distinct bands.
Thus, PFGE makes it possible to separate and analyze extremely large DNA molecules, which are otherwise inseparable using standard gel electrophoresis.
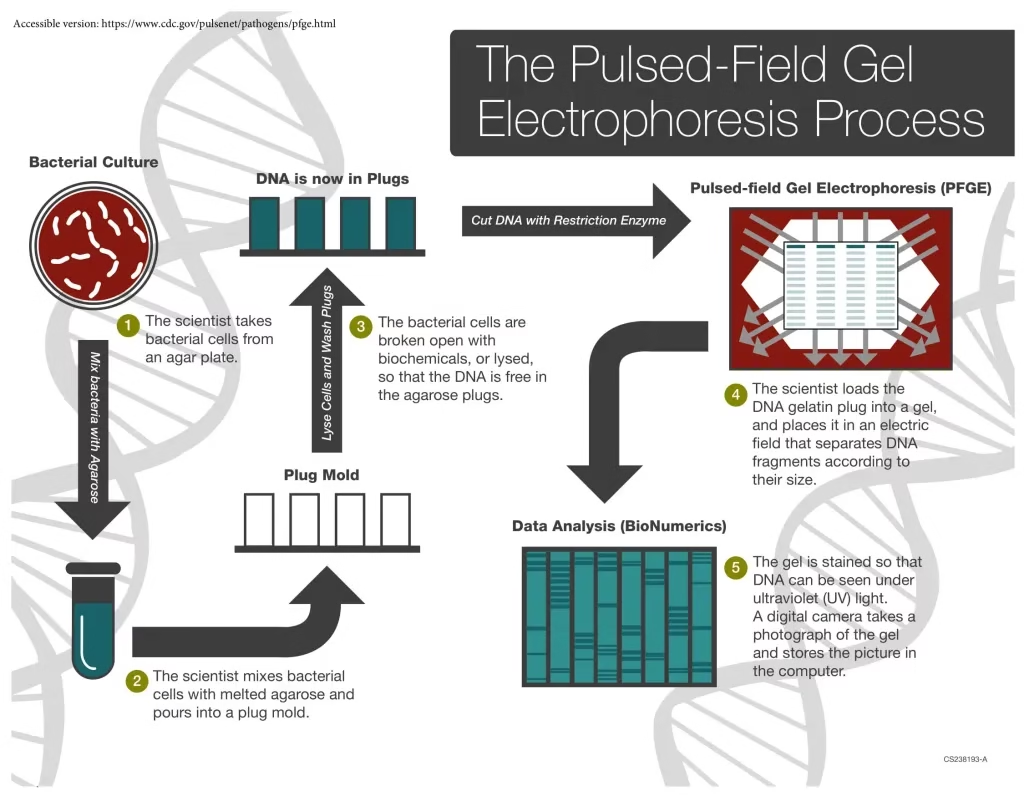
Procedure of Pulsed Field Gel Electrophoresis (Step-by-Step)

The PFGE procedure is similar to conventional gel electrophoresis, but with pulsed electric fields instead of a constant one. The key steps are:
1. Preparation and Lysis
- Bacterial cells are suspended in molten agarose to form small blocks called plugs.
- Embedding DNA in agarose protects it from mechanical shearing.
- The bacterial cells in the plugs are lysed, releasing intact chromosomal DNA.
2. Digestion of DNA
- The DNA is treated with rare-cutting restriction enzymes.
- Unlike common enzymes that cut frequently, these enzymes produce fewer, very large DNA fragments.
- Example enzymes: NotI, SmaI, XbaI.
3. Electrophoresis
- The DNA-containing plugs are placed in an agarose gel.
- An electric current is applied in three directions:
- One along the gel’s central axis
- Two at 60° angles on either side
- The current alternates direction at set intervals (pulse times).
- This creates a net forward migration of DNA fragments, with separation based on size.
4. Analysis
- After electrophoresis, the gel is stained (commonly with ethidium bromide or SYBR Green) to visualize DNA bands.
- The DNA patterns are analyzed manually or using specialized software like BioNumerics.
- Comparison of banding patterns allows strain typing and genetic fingerprinting.

Applications of Pulsed Field Gel Electrophoresis
PFGE has a wide range of applications in microbiology, medicine, and molecular biology:
- Chromosome Mapping
- PFGE helps create physical maps of bacterial chromosomes, especially for large DNA fragments.
- Epidemiological Studies
- Considered the gold standard for bacterial subtyping.
- Used in outbreak investigations to identify the source of infection.
- Food Microbiology
- Helps track contamination sources in outbreaks caused by Listeria monocytogenes, Salmonella, and E. coli O157:H7.
- Clinical Microbiology
- Differentiates between strains of pathogens.
- Helps link environmental isolates to clinical cases.
- Public Health Surveillance
- Used by organizations like CDC (Centers for Disease Control and Prevention) in the PulseNet program for foodborne pathogen surveillance.
- Molecular Genetics
- Useful for studying genome structure and large-scale genetic rearrangements.
Advantages of Pulsed Field Gel Electrophoresis
- Can separate DNA fragments from a few kb up to >10 Mb.
- Highly discriminatory – differentiates between strains of the same species.
- Provides stable and reproducible DNA restriction patterns.
- Acts as a universal method for subtyping many bacteria (just need to adjust enzyme and conditions).
- More accurate than ribotyping or multilocus sequence typing for many bacterial species.
Limitations of Pulsed Field Gel Electrophoresis
- Time-consuming (2–5 days to complete an assay).
- Requires skilled technicians and expensive equipment.
- Cannot always distinguish between unrelated isolates with similar patterns.
- Results may vary slightly between laboratories or operators.
- Bands of the same size may not represent identical DNA sequences.
- Some bacterial strains are non-typeable by PFGE.
- Not ideal for very small DNA fragments (<20 kb).
Summary
- PFGE is an advanced electrophoresis technique designed to separate extremely large DNA fragments.
- Works by applying alternating electric fields that force DNA to reorient, enabling size-based separation.
- Plays a crucial role in epidemiology, pathogen typing, chromosome mapping, and food safety.
- Despite being time-consuming, PFGE remains a gold standard for bacterial genotyping and outbreak investigations.
Frequently Asked Questions (FAQs) on Pulsed Field Gel Electrophoresis (PFGE)
Q1. What is Pulsed Field Gel Electrophoresis (PFGE)?
Ans: PFGE is an advanced form of gel electrophoresis used to separate very large DNA fragments (up to several megabases) that cannot be resolved by standard agarose gel electrophoresis.
Q2. Who invented PFGE and when?
Ans: PFGE was developed in 1982 by Schwartz and Cantor.
Q3. What is the principle of PFGE?
Ans: PFGE separates DNA fragments by applying alternating electric fields. Large DNA molecules take longer to reorient, while smaller fragments realign quickly and migrate faster, leading to separation.
Q4. What size range of DNA can PFGE separate?
Ans: PFGE can separate fragments from 50 kb to more than 10 Mb.
Q5. Why can’t standard agarose gel electrophoresis separate large DNA fragments?
Ans: In normal gels, DNA fragments larger than ~20–30 kb migrate at the same rate and form a single band, making them indistinguishable.
Q6. What are agarose plugs in PFGE?
Ans: DNA is embedded in agarose plugs to prevent shearing during handling, ensuring intact large DNA molecules.
Q7. Which restriction enzymes are commonly used in PFGE?
Ans: Rare-cutting restriction enzymes such as NotI, XbaI, SmaI, and SpeI are commonly used to generate large DNA fragments.
Q8. What equipment is required for PFGE?
Ans: A PFGE system includes an electrophoresis chamber, cooling system, power supply capable of alternating fields, and software for band analysis.
Q9. How long does PFGE take?
Ans: A typical PFGE run takes 2–5 days, depending on DNA size and conditions.
Q10. What is PulseNet?
Ans: PulseNet is a network coordinated by the CDC (Centers for Disease Control and Prevention) that uses PFGE to track foodborne bacterial pathogens.
Q11. Why is PFGE called the “gold standard” for epidemiology?
Ans: Because PFGE provides highly reproducible, discriminatory, and reliable DNA fingerprinting patterns used in outbreak investigations worldwide.
Q12. What are the applications of PFGE in microbiology?
Ans: PFGE is used for:
- Epidemiological typing of bacteria
- Food safety testing
- Chromosome mapping
- Public health surveillance
- Clinical outbreak investigations
Q13. Can PFGE distinguish between strains of the same bacterial species?
Ans: Yes. PFGE is highly discriminatory and can differentiate strains within the same species, which is why it’s widely used in epidemiology.
Q14. What are the advantages of PFGE?
Ans: PFGE allows:
- Separation of very large DNA fragments
- High reproducibility
- Universal application across many bacteria
- Effective outbreak tracking
Q15. What are the limitations of PFGE?
Ans: PFGE is time-consuming, expensive, labor-intensive, requires skilled staff, and sometimes fails to distinguish unrelated isolates with similar patterns.
Q16. Can PFGE be automated?
Ans: Partial automation exists with software-based analysis tools (like BioNumerics), but the electrophoresis step itself is still slow and manual.
Q17. Is PFGE useful in viral studies?
Ans: PFGE is mostly used for bacterial DNA and not ideal for small viral genomes, which are better studied with sequencing techniques.
Q18. What staining methods are used in PFGE?
Ans: DNA fragments are visualized using stains such as ethidium bromide (EtBr) or SYBR Green.
Q19. Can PFGE results be compared between laboratories?
Ans: Yes, but requires standardized protocols (e.g., PulseNet protocols) to ensure reproducibility.
Q20. How does PFGE compare with Whole Genome Sequencing (WGS)?
Ans: PFGE is cheaper and faster for outbreak screening, but WGS provides higher resolution and more detailed genetic information. Many labs now use PFGE alongside WGS.
Q21. Why is cooling important during PFGE?
Ans: Large DNA fragments generate heat during long electrophoresis runs, so constant cooling prevents gel distortion and maintains resolution.
Q22. Is PFGE still used today?
Ans: Yes. PFGE is still widely used in foodborne outbreak investigations, epidemiology, and chromosome mapping, though Whole Genome Sequencing (WGS) is gradually replacing it.
References
- Bailey, W. R., Scott, E. G., Finegold, S. M., & Baron, E. J. (1986). Bailey and Scott’s Diagnostic microbiology. St. Louis: Mosby.
- Sastry A.S. & Bhat S.K. (2016). Essentials of Medical Microbiology. New Delhi : Jaypee Brothers Medical Publishers.
- Parija S.C. (2012). Textbook of Microbiology & Immunology.(2 ed.). India: Elsevier India.
- https://www.slideshare.net/pankajgaonkar31/pulse-field-gel-electrophoresis
- https://en.wikipedia.org/wiki/Pulsed-field_gel_electrophoresis
- https://microbenotes.com/pulsed-field-gel-electrophoresis-pfge/
- https://bitesizebio.com/29971/pulsed-field-gel-electrophoresis/
Other related topics you might be interested in:
Agarose Gel Electrophoresis – Principle, Procedure, Applications, Advantages & Limitations